INTRODUCTION
Obstructive sleep apnea/hypopnea syndrome (OSAHS) is characterized by transient upper airway resistance caused by a recurrent reduction or cessation of airflow, due to partial or complete occlusion of the upper airway during sleep. It is associated with sleep fragmentation, arousals, bradycardia, tachycardia, and inadequate oxygen saturation despite an increased respiratory effort(1). Major clinical consequences of the disorder include excessive daytime sleepiness, neurocognitive dysfunction, cardiovascular diseases (hypertension, stroke, myocardial infarction, and heart failure), and metabolic dysfunction(2). Risk factors include obesity, male gender, thick neck, upper respiratory tract abnormalities, and excessive alcohol intake(3). Additionally, OSAHS has been shown to affect the vascular endothelium by promoting inflammation and oxidative stress; vascular autoregulation with high sympathetic stimulus increases the time delay of the baroreflex response of muscle sympathetic nerve activity(4,5).
The choroidal vasculature provides the major blood supply to the outer retina, and plays an important role in temperature control and volume regulation. The smooth muscle of the choroid vessel walls are innervated by sympathetic and parasympathetic nerves. It has been shown that choroidal blood flow is autoregulated in order to maintain adequate oxygen saturation and to keep the temperature on the retina constant, despite changes in systemic blood flow(6). Previous studies have suggested relationships between OSAHS and several ocular diseases, including central serous chorioretinopathy, glaucomatous optic neuropathy, and ischemic optic neuropathy(7-9). It has been speculated that the inflammation, catecholamine excretion, and raised sympathetic activity and blood pressure triggered by OSAHS may play a role in the pathogenesis of these diseases(10,11).
Optical coherence tomography (OCT) is a noncontact imaging method that allows for repetitive, high resolution cross-sectional imaging of the retina and choroid, and enables the evaluation of living choroid. In 2008, Spaide et al., reported the technique of enhanced depth imaging (EDI) OCT (820-nm wavelength), which is capable of obtaining images from deep layers of the retina. This has enabled the technique to be used to investigate choroidal diseases such as central serous chorioretinopathy, age-related macular degeneration, and polypoidal choroidal vasculopathy(12-14).
In this study, we aimed to compare the subfoveal choroidal thickness (SFCT) of patients with OSAHS and control subjects using EDI-OCT. Also, the study examined whether a relationship exists between different clinical grades of OSAHS and choroidal thicknesses.
METHODS
Sixty-two patients who presented with problematic snoring and daytime sleepiness were admitted for an overnight polysomnographic evaluation of suspected OSAHS. They were subsequently referred for ophthalmologic evaluation at the ophthalmology clinic at Konya Education and Research Hospital between June 2011 and July 2012. This retrospective, case-control study was conducted according to the tenets of the Declaration of Helsinki and with the approval of the ethics committee of Selcuk University. All OSAHS subjects underwent a standard overnight polysomnography. This investigation was performed using the Compumedics E-series Sleep System, (Compumedics, Melbourne, Australia). Electroencephalography (EEG), submental electromyography (EMG), electrooculography (EOG), leg EMG, and electrocardiography (ECG) recordings were obtained. In addition, air-flow was measured using both a nasal cannula (NC) and a nasal thermistor, oxygen saturation (SaO2) was measured by a pulse oximeter, and chest and abdominal respiratory movements were monitored. A decrease in oxygen saturation of less than 4% or the occurrence of symptoms of physiologic awakening following at least a 30% reduction in air flow for a minimum of 10 seconds, was defined as hypopnea. Individuals with an apnea hypopnea index (AHI) ≥5 were diagnosed as OSAHS and were graded according to the following AHI values: mild, 5≤AHI<15; moderate, 15≤AHI<30; severe, AHI≥30. Patients who had an AHI<5 were included as control subjects.
Ophthalmic examinations were conducted, including best-corrected visual acuity, slit-lamp biomicroscopy, Goldman applanation tonometry, automated visual field (VF) examination, and fundoscopy. All patients had a best-corrected visual acuity of 20/20 or higher. Exclusion criteria included a history of chronic or recurrent inflammatory eye diseases (e.g., scleritis, uveitis), other confounding chorioretinal pathologies, any abnormality preventing reliable applanation tonometry, signs of glaucoma, a history of intraocular trauma, unstable and uncontrolled cardiovascular, renal, or pulmonary diseases, diabetes, pregnancy, and spherical and cylindrical refractive errors of more than ± 3.0 diopters. We reviewed the medical records and OCT images of patients who were especially examined for retinal nerve fiber layer (RNFL) defects in relation with glaucoma, and a macular OCT scan was performed in order to exclude other pathologies.
OCT images were obtained in the EDI mode of a spectral domain OCT (Spectralis, Heidelberg Engineering, Heidelberg, Germany). For EDI-OCT, foveal-centered vertical and horizontal two line scans were performed in an assay of 100 frames, 30°, and high resolution (HR). SFCT was calculated by obtaining mean measurements of the vertical and horizontal scans thicknesses. The choroidal thickness was manually measured via the software in the OCT device with magnified images (× 200). The distance from the hyperreflective line at the base of the retina pigment epithelium layer to the hyporeflective line in the outer sclerochoroidal interface was accepted as the choroidal thickness (Figure 1). When necessary, contrast assays were altered to obtain improved images of the choroidal layer. Pupils were not dilated for the test. Recordings with a quality (Q) below 20 were excluded. Choroidal thickness evaluations were performed by a single examiner (MO) who was blinded to patient diagnoses. The left eye of each patient was tested if not accompanied by exclusion criteria.
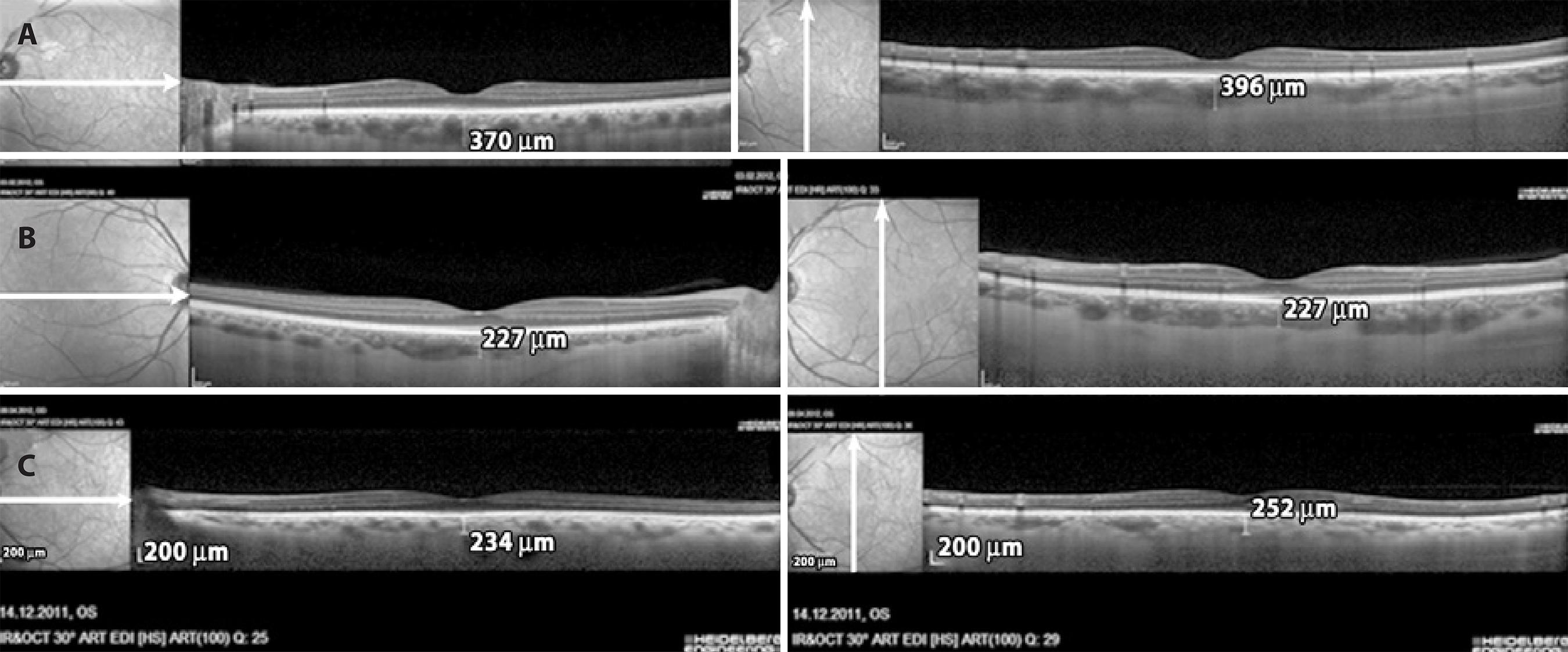
Figure 1 Measurements of subfoveal choroidal thickness using the EDI mode of spectral domain OCT (Spectralis, Heidelberg Engineering, Heidelberg, Germany). A) A mild OSAHS patient; B) A moderate OSAHS patient; C) A severe OSAHS patient.
Statistical analysis
Data were analyzed using IBM SPSS; version 13.0 for Windows (SPSS Inc., Chicago, Illinois, U.S.). Continuous variables were presented: average, standard deviation, and median [interquartile range]. We performed two separate analyses. First, patients were divided into four groups; without OSAHS (control), mild OSAHS, moderate OSAHS, and severe OSAHS. Second, to determine whether OSAHS severity influenced the SFCT, we divided the patients into three groups; without OSAHS (control), mild OSAHS, and moderate/severe OSAHS (15≤AHI). After the assessment of normality assumption, we used one-way analysis of variance (ANOVA) to analyze the differences in measurement values between the groups. The Bonferroni correction was applied when necessary for multiple comparisons. P<0.017 was considered to be significant after Bonferroni correction. The Mann-Whitney U test and Kruskal-Wallis test were used to analyze the BMI and AHI values. The correlation between AHI and SFCT was evaluated using the Spearman's correlation test, and the correlation between age and SFCT was evaluated using Pearson's correlation coefficient test. A Paired-samples t-test was used to compare horizontal and vertical line scan SFCT measurements. P<0.05 was regarded as statistically significant.
RESULTS
The recordings of 62 patients were assessed and 13 (20%) were excluded from the study. Of these, five were excluded because of diabetic retinopathy, three because of glaucoma, two because of high spherical and astigmatic refraction, and the other three because of age-related macular degeneration, amblyopia, and OSAHS treatment. Thus, a total of 49 subjects were included in the study, of which 35 were OSAHS patients and 14 were non-OSAHS controls. Patients were divided into four groups according to the AHI: control group, 14 patients (AHI<5); mild OSAHS group, 15 patients (AHI 5-15); moderate OSAHS group, 11 patients (AHI 15-30); and severe OSAHS group, 9 patients (AHI≥30). Patient ages and intraocular pressures (IOP) between the groups were not significantly different (P=0.544 and 0.995, respectively). Table 1 summarizes the patients' demographic data. Despite a slight, but not significant, chorodial thinning observed in the severe group, the SFCT values between the control and severe groups, mild and severe groups, and moderate and severe groups, revealed no significant differences (P=0.09, 0.04, and 0.87, respectively; Bonferroni correction P<0.017; table 2). The moderate and severe groups were then merged and a second statistical analyses was performed. Again, no significant differences in age or IOP values was found between the groups (P=0.400 and 0.984, respectively). The mean subfoveal choroidal thickness was 314.5 ± 83.3 μm in the control group, 324.5 ± 6 μm in the mild group, and 267 ± 52.8 μm in the moderate/severe group (Table 3). The difference between the SFCT values of the control and mild groups was not statistically significant (P=0.693). However, the difference between the SFCT values of the control and moderate/severe groups approached significance (P=0.05), and SFCT of the mild group was significantly thinner than that of the moderate/severe group (P=0.016) (Table 3). In addition, we found a negative correlation between SFCT and AHI in OSAHS patients (r=0.368, P=0.033). Also, our results show a statistically significant negative correlation between patient age and SFCT (r=0.422, P=0.003). We did not find a significant difference in the SFCT measurements of the horizontal and vertical line scans (P=0.683).
Table 1 Demographic data of controls and OSAHS patients according to the AHI
| Mean ± standard deviation median (interquartile range) | Control | Mild OSAHS | Moderate OSAHS | Severe OSAHS | p-value* | |
|---|---|---|---|---|---|---|
| Number of patients | 14 | 15 | 11 | 9 | ||
| Age (years) | 43.4 ± 9.5 | 45.4 ± 6.7 | 46.6 ± 9.5 | 48.8 ± 9.7 | ap=0.544 | |
| Sex | ||||||
| Male | 2 | 6 | 8 | 6 | ||
| Female | 12 | 9 | 3 | 3 | ||
| IOP (mmHg) | 14.9 ± 2.2 | 14.9 ± 2.5 | 15.1 ± 1.6 | 14.9 ± 2.3 | ap=0.995 | |
| 15 (3) | 15 (2) | 15 (1) | 15 (4) | |||
| BMI (kg/m2) | 29.7 ± 3.9 | 30.9 ± 4.7 | 30.7 ± 8.4 | 35.2 ± 3.3 | bp<0.050 | |
| 28.7 (4) | 29.7 (7.1) | 28.9 (13.5) | 35.9 (5.9) | |||
| AHI (hours) | 2.1 ± 1 | 8.6 ± 3.2 | 19.9 ± 2.9 | 64.5 ± 27.3 | bp<0.001 | |
| 1.8 (1.6) | 7.6 (4.9) | 20.2 (4.2) | 59.2 (42.4) | |||
AHI= apnea-hypopnea index; OSAHS= obstructive sleep apnea/hypopnea syndrome; BMI= body mass index; IOP= intraocular pressure.
*= ap- ANOVA; bp- Kruskal Wallis test.
Table 2 Comparison of SFCT (μm) measurements between control, mild (5≤AHI<15), moderate (15≤AHI<30) and severe (AHI≥30) OSAHS patients
| Mean ± standard deviation | Control | Mild OSAHS | Moderate OSAHS | Severe OSAHS | p-value* |
|---|---|---|---|---|---|
| Subfoveal choroidal thickness | 314.5 ± 83.3 | 324.5 ± 69 | 269.3 ± 60.4 | 264.3 ± 45.2 | 0.08 |
AHI= apnea-hypopnea index; OSAHS= obstructive sleep apnea/hypopnea syndrome; SFCT= subfoveal choroidal thickness.
*= ANOVA.
Table 3 Comparison of SFCT (μm) measurements between controls, mild and Moderate/Severe OSAHS patients
| Mean ± standard deviation | Control | Mild OSAHS | Moderate/severe OSAHS patients | p-value * |
|---|---|---|---|---|
| Subfoveal choroidal thickness | 314.5 ± 83.3 | 324.5 ± 6 | 267 ± 52.8 | p=0.033 |
OSAHS= obstructive sleep apnea/hypopnea syndrome; SFCT= subfoveal choroidal thickness.
*= ANOVA.
DISCUSSION
In this study, we evaluated the SFCT of patients suffering from OSAHS, which is associated with intermittent upper airway obstruction during sleep. We found that the SFCT measurements of the moderate/ severe OSAHS group were significantly lower than the mild OSAHS group and were nearly significantly lower than the control group. We also observed a negative correlation between AHI and SFCT, and between age and SFCT, in patients with OSAHS.
The choroid is a microcirculatory vascular structure and its thickness and blood flow depend on the perfusion pressure, intraocular pressure, nitric oxide production, endogenous catecholamines, and vascular autoregulation(15-18). The subfoveal region is the thickest part of the choroid and it provides for the needs of the fovea, which has the highest photoreceptor density and metabolic activity(19).
Stable choroidal layer perfusion pressure is associated with vascular resistance controlled by the sympathetic nervous system(20). Released from the vascular endothelium, endothelin-1 and nitric oxide (NO) also play a role in the adaptation of choroidal vascular resistance(18,21,22). The choroidal layer has the ability to regulate ocular perfusion pressure (which is 67% above baseline)(21,23). It has been shown that stable perfusion is produced by ocular vasoconstriction(20). This autoregulation is very important for outer retinal oxygenation and the control of macular temperature.
There is some evidence that the metabolic effects of OSAHS lead to the activation of oxidative stress, endothelial dysfunction, pro-inflammatory agent secretions, and adrenergic system induction(24). Also, OSAHS may result in a poorer response to metabolic stress conditions. However, Khayi et al. demonstrated that in OSAHS patients, choroidal blood flow is appropriately autoregulated when ocular perfusion pressure is increased by exercise(25). Recently, several studies have reported that OSAHS is associated with central serous chorioretinopathy, glaucomatous optic neuropathy, and ischemic optic neuropathy(7-9). OSAHS and central serous chorioretinopathy share a similar etiopathogenesis, including stress and an overproduction of catecholamines and inflammatory agents, which can affect choroidal regulation(11). In patients with central serous chorioretinopathy, EDI-OCT has demonstrated an association with a thicker subfoveal choroidal layer(7).
In addition, an association between a diagnosis of OSAHS and an increased risk of open angle glaucoma has previously been demonstrated(26), and it has also been shown that RNFL thickness is reduced in OSAHS patients, even though there are no visual field defects(27). Also, the subclinical progression of ischemic optic neuropathy may be associated with choroidal layer pathology. It has previously been suggested that the association between OSAHS and ocular disease is due to the OSAHS-induced metabolic effect of hypoxemia on systemic and local ocular vascular dysregulation(7-9). The signs of these metabolic effects on the choroidal layer may prove to be valuable for the risk evaluation of ocular diseases, and in their follow up.
The results of our study are consistent with those of Xin et al., who showed that the SFCT of a severe OSAHS group was significantly thinner than that of a control group and mild and moderate OSAHS groups. SFCT difference between our moderate/severe group and control group approached significance. This might be due to the origin of the control group that has some suspicious symptoms related with possible sleep disorders, and the small number of subjects. Also, this study revealed a negative correlation between AHI severity and SFCT(28). However, OSAHS is a risk factor for central serous chorioretinopathy etiology, which has been reported to accompany subfoveal choroidal effusion(7). Khayi et al., suggest that the regulation of ocular blood flow, which partially depends on the sympathetic and parasympathetic systems, is not altered without comorbidities associated with OSAHS(25). However, it has been shown that there is an increased risk of cardiovascular and pulmonary diseases in OSAHS patients(29). An increased risk of glaucoma in OSAHS patients may also be associated with additional pathologies that are compatible with the vascular theory of glaucoma. Apneas involving hypoxia and low systemic diastolic tensions may affect ocular perfusion and oxygenation, particularly in the presence of accompanying pathologies such as anemia and cardiovascular and pulmonary diseases. It is evident that further prospective studies are needed, especially relating to ischemic optic pathologies and OSAHS.
EDI, a new feature of OCT, has allowed us to accurately assess the choroidal layer structure in vivo. We measured subfoveal choroidal thickness manually with spectralis OCT, and we concede that the manual nature of the measurements has the potential to result in errors. However, there is no available software to provide automatic measurements. We found no statistical difference between vertical and horizontal line scan measures of the same location, which verifies the accuracy of our measurements (P>0.05). As further support for our measurement results, studies have been published that show a high reproducibility and repeatability of manual measurements of choroidal thickness(30). Additionally, we paid special attention to select patients who had good choroidal layer images, which strengthened the measurement reliabilities.
This study has some important limitations. It was a retrospective study of a small sample size, particularly after the OSAHS patients had been divided into subgroups according to their severity. In addition, choroidal thickness grading was only performed by one examiner, and we were not able to determine the smoking habits of the patients. Furthermore, OSAHS duration, which has been shown to influence vascular autoregulation ability(31), was unknown. Another limitation was that our control group was not symptom-free, so they were required to undergo a standard overnight polysomnography. This was important in order to determine the presence/absence and severity of sleep apnea/hyponea. However, it may also have resulted in an unexpected selection bias.
CONCLUSION
SFCT was significantly thinner in patients with moderate/severe OSAHS than in patients with mild OSAHS. The AHI was negatively correlated with SFCT of all OSAHS patients. Our results suggest that vascular changes associated with OSAHS can be evaluated by measuring SFCT, via noninvasive EDI-OCT. Further studies are needed to determine more accurately whether there is a relationship between clinical grades of OSAHS and choroidal thickness.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

