INTRODUCTION
Posterior capsule opacification (PCO) is one of the most common postoperative complications following cataract surgery(1,2). It usually leads to a decrease in visual acuity (VA), a loss of contrast sensitivity, and glare disability. It also precludes examination of the posterior segment(3). Patients who have visual function reduction in response to PCO may be treated with Neodymium (Nd):YAG laser capsulotomy to restore VA, but this treatment is not free of complications(1,4).
Researchers are trying to identify the key factors in reducing the incidence of PCO(5,6). It has already been demonstrated that sharp-edged intraocular lens (IOL) optics are preferable to round-edged IOL optics(7). Modification of the IOL surface, which can inhibit cell and protein adhesion, has been suggested for reducing the incidence PCO(4). Furthermore, modifications of IOL design, maintaining an open capsular bag, enhancing the aqueous endocapsular inflow, and even the use of a bag-in-the-lens technique, all appear to prevent capsular bag opacification(8,9).
The causes of opacification include cell proliferation and migration, epithelial-to-mesenchymal cell transitions, collagen deposition, and lens fiber regeneration of lens epithelial cells(10). The main factors relating to PCO development are either patient related (e.g., the age and type of ocular disease), surgery related (e.g., the capsulorrhexis size, and whether irrigation/aspiration of the capsule, a hydrodissection-enhanced cortical clean-up, sealed capsule irrigation, and in-the-bag IOL fixation, are performed), and IOL related (material and design of the implanted IOL)(8,10-12). The Ioflex (Mediphacos) is a 1-piece foldable acrylic hydrophilic IOL for posterior chamber implantation, designed with square edges for PCO prevention. It has a low cost and has been marketed for over ten years in several European, Asian, and Latin American countries. Its postoperative performance has not yet been studied. Thus, this study aimed to evaluate the incidence of PCO four years after Ioflex IOL implantation.
METHODS
A sample of 150 eyes was randomly selected. This sample size calculation was based on the PCO incidence reported in the literature. The patients were from a population that had undergone cataract surgery and Ioflex IOL implantation in 2007, as part of a community campaign for underprivileged people. A total of 50 eyes (31 patients) could not be located, either due to communication difficulties relating to geographical distance, or to patient death. Of the 100 eyes located, 13 were not presented for examination. Thus, 87 eyes corresponding to 58 patients, were available for examination. However, to avoid bias, in patients who underwent cataract surgery in both eyes, only the right eye was included in the study. Therefore, a final total of 58 eyes from 58 patients were submitted to a detailed ophthalmic examination.
The inclusion criteria comprised being subjected to phacoemulsification and Ioflex IOL implantation between January and June 2007. The exclusion criteria comprised intraoperative complications, such as posterior capsule rupture, and non-Ioflex IOL implantation. The same surgeon performed all surgical procedures using a Universal II Phacoemulsifier (Alcon). In each case a 2.8 mm incision was made under peribulbar anesthesia, using balanced salt solution (BSS®, Alcon) and 2% methylcellulose viscoelastic (Ophthalmos). Identical continuous curvilinear capsulorrhexis and cortical clean-up procedures were used in all cases. The ophthalmic examination included measurements of corrected distance visual acuity (CDVA) and biomicroscopy slit lamp evaluations after pupil dilation with Mydriacyl (Alcon). Patients with a VA decreased in response to PCO were referred to Nd:YAG laser treatment. A detailed medical history for each patient was also obtained. The patients' charts were reviewed for their CDVA prior to IOL implantation as well as for one month postoperatively.
This study was approved by an Ethics Committee (CEP: 054/2011) and followed the Declaration of Helsinki principles. The variables were expressed as the mean and standard error of the mean. We used the Student's t-test for independent samples to check for possible differences in VA, and the Student's t-test for paired samples to analyze the mean VA of patients with and without PCO during the evaluation periods. A p value of <0.05 was adopted for rejection of the null hypothesis.
RESULTS
Of the 58 patients, 27 were male and 31 female. The mean age of patients without PCO in the four year follow-up period was 74.6 ± 9.5 years, and the mean age of patients with PCO over the same period was 70.3 ± 15.1. There was no statistically significant difference between the mean age of the patients with or without PCO (p=0.182). All surgeries were uneventful both intra and postoperatively.
There was no statistically significant difference between the mean VA of the patients with or without PCO during the evaluation period. Preoperative CDVA ranged from hand movement to 0.30 logMAR. One month after cataract surgery with Ioflex IOL implantation, improvement of VA was observed in all but two patients. These two patients either had glaucoma or an age-related macular degeneration. On systemic disease evaluation, 45% (26 patients) reported systemic arterial hypertension and 3.5% (2 patients) had diabetes mellitus.
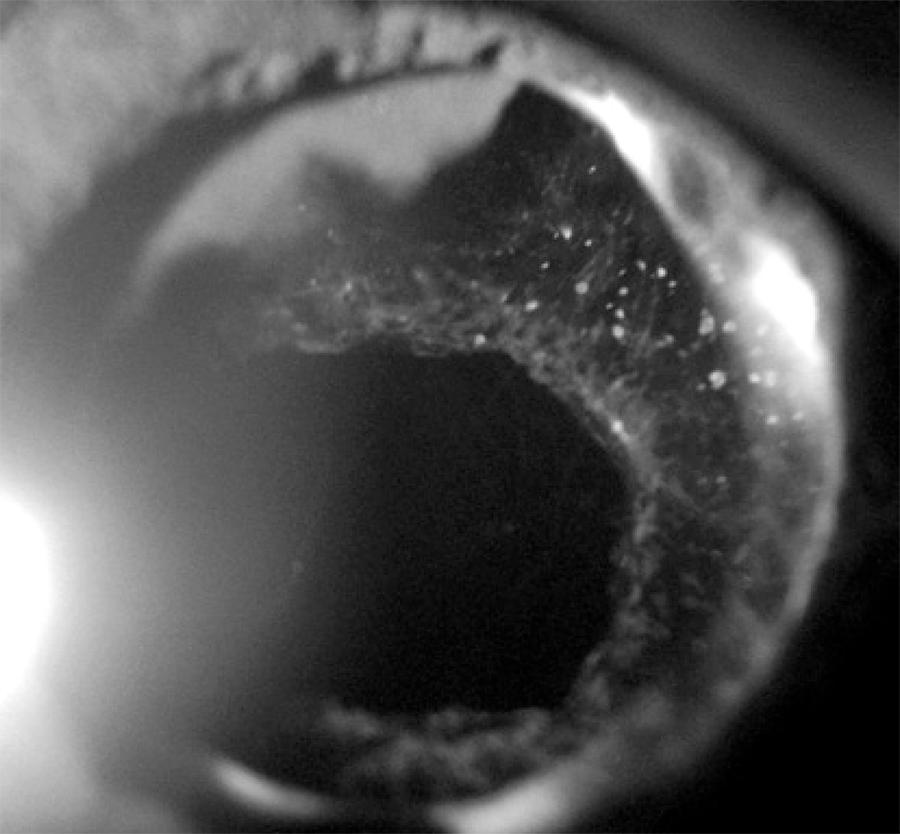
Four years after surgery, 39 out of 58 patients (67%) had PCO as detected by a slit lamp examination. Of the total, 24 eyes (41.3%) had decreased VA due to PCO and were referred for Nd:YAG laser capsulotomy (Figure 1). Three eyes (5.1%) had decreased VA due to glaucoma, IOL opacification, and age-related macular degeneration, respectively. In a further 12 eyes (20.7%) with mild PCO, VA remained unchanged.
DISCUSSION
This study aimed to evaluate the PCO rates in Ioflex IOLs four years after surgery. We found that 67% of patients suffered from this postoperative complication. Clinical studies have shown varied incidences of PCO for other hydrophilic IOLs, ranging from 4.6% to 50%(1,13-15). Notably, all of these incidence rates are less than the one we have found. To our knowledge, there are no other published studies that have reported the incidence of PCO in the Ioflex IOL. As the eradication of PCO is a major goal of researchers working to improve IOL materials and design, as well as surgical techniques(6,10,16), it is important that additional research is performed.
The IOL material and design is able to influence the incidence of PCO(5,7,8). It has been well documented that a hydrophobic lens material causes less PCO than a hydrophilic material, even though the latter is more uveal biocompatible(17,18). However, PCO may progresses significantly with time and is more extensive following polymethylmetacrylate IOL implantation, while its progression rate after silicone and acrylic IOL implantation is low(19).
Previous reports have shown that hydrophilic IOLs lead to at least twice the number of Nd:YAG laser treatments when compared with hydrophobic lenses(1,13,17,18). Although Nd:YAG laser capsulotomy is the standard treatment for PCO and is generally found to be safe and effective, it is also expensive and not free of complications(1,11,20), including a significant rate of morbidity from postoperative complications. The most common complication is intraocular pressure elevation, while other complications include cystoid macular edema, retinal hemorrhage or detachment, iritis, vitreous prolapse, corneal damage, vitritis, iris damage, pupillary blockage, hyphema, IOL subluxation, and localized exacerbation of endophthalmitis(21,22). PCO is also associated with socioeconomic factors, since an outpatient examination is required to diagnose it and, depending on local policies, an additional hospital visit may be required for Nd:YAG laser treatment(23). All of these factors emphasize the importance of efforts aimed at minimizing Nd:YAG laser capsulotomy necessity.
Following technological advances, IOL design has been modified from round-edged to square-edged, and this has resulted in a reduction in the incidence of PCO(24). However, studies suggest that square-edged IOLs should completely encompass the 360 degrees around the IOL optic in order to provide an effective barrier(25). Furthermore, another study has shown differences between brands of square-edged lenses, and suggested that variations in their edge profiles may account for clinical differences in postoperative PCO rates(26). In addition, it has been reported that acrylic IOLs appear to lose their PCO preventive effect, despite their sharp optic edges(27).
Although the Ioflex is a 1-piece lens, this not considered to be a factor in the development of PCO, since both 1- and 3-piece IOLs have been studied to evaluate their influence on PCO formation and no statistically significant differences between them have been found(28,29).
PCO is also age-dependent, being more frequently observed as a postoperative complication in young people and children(7,30). This study evaluated an adult population and thus did not find a statistically significant difference on the independent analysis of age between patients with and without PCO.
Decreased VA induced by PCO was noted in 41% of patients. This value is supported by data in the published literature, and confirms that it represents the most frequent cause of vision reduction following cataract surgery(7,15). Only one patient presented IOL opacification, but we had previously reported, in another study with the same IOL, an incidence as high as 7% of long term IOL opacification, with no correlation to PCO(16).
CONCLUSION
This study found a PCO incidence of 67% four years after Ioflex IOL surgery. This rate is higher than that reported for other hydrophilic lenses described in the literature. A better understanding of the pathogenic mechanisms of PCO is highly desirable as a basis for improving the outcome of cataract surgery and for eradicating this serious postsurgical complication.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

