INTRODUCTION
Pterygium is characterized by the encroachment of a fleshy fibrovascular tissue from the bulbar conjunctiva onto the cornea. Although historically described as a degenerative disorder, it is more closely associated with inflammation and progressive fibrovascular proliferation(1-3).
The pathogenesis of pterygium appears to be complex. Despite being historically described more as a degenerative process, inflammation and fibrovascular proliferation are currently proven to be important factors. Angiogenesis has also been demonstrated during pterygium formation and progression(4-5).
The current treatments for pterygium focus on surgical excision and prevention of recurrence. The extent and severity of the fibrovascular growth of pterygium seem to comprise a reliable morphological index for predicting recurrence after surgery(6).
Recurrence of pterygium is the most common undesirable outcome after surgical excision. Recurrent pterygia are more hazardous than primary ones because the underlying cornea may be thinner. Extensive scarring from previous procedures can adversely affect visual acuity, and further recurrence is common(7).
Several studies have shown that the increased expression of basic fibroblast growth factor (bFGF), transforming growth factor (TGF-β), vascular endothelial growth factor (VEGF), and platelet-derived growth factor correlates with the formation and recurrence of pterygia(8-11).
Bevacizumab (Avastin®, Genentech, Inc., San Francisco, CA, USA) is a humanized monoclonal antibody to VEGF designed for intravenous (IV) administration and approved for the treatment of colorectal cancer(11). Various clinical trials have shown that intravitreal administration is well tolerated and associated with an improvement in visual acuity, decrease in central retinal thickness, and decrease in angiographic leakage(12,13).
Pterygia are composed of proliferating fibrovascular tissue, and pterygium formation and progression require new blood vessel formation(14). Prominent regression of limbal-conjunctival neovascularization and delayed recurrence have been reported with the use of topical bevacizumab in patients with impending recurrent pterygium(15-16).
Anti-VEGF therapy may potentially suppress neovascularization in pterygium, preventing or retarding the progression of recurrence(17-19). This study aimed to determine the clinical effectiveness and safety of subconjunctival injection of bevacizumab for recurrent pterygium.
METHODS
This was a prospective, single-dosing, interventional case series conducted at Instituto de Olhos de Goiânia (Goiânia Eye Institute), a hospital in Goiânia (Brazil), from March 2012 to December 2012 in patients with recurrent pterygium. Recurrent pterygium was defined as the growth of fibrovascular tissue over the limbus onto the clear cornea in the area of previous pterygium excision.
The pterygia were graded according to the system used by Tan et al.(6): grade I (atrophic), in which the episcleral vessels under the body of the pterygium are not obscured and clearly distinguished; grade II (intermediate); and grade III (fleshy), in which the episcleral vessels are totally obscured. Exclusion criteria included any condition for which bevacizumab was contraindicated (allergy to bevacizumab, proteinuria, bleeding tendencies, previous myocardial infarction or stroke, pregnancy, and lactation), evidence of other ocular diseases except refraction errors, use of topical medications for pterygium, presence of other complaints not attributable to pterygium, prior ocular trauma, more than one recurrent pterygia, and the inability to follow-up for the entire duration of the study.
Patients were interviewed before injection using a questionnaire to obtain information such as general data, contact number, demographic factors, and medical, surgical, and ocular history.
Informed consent was obtained from all patients after the nature and possible consequences of the study were explained. The study was approved by the Institutional Research Ethics Committee. In cases of bilateral recurrent pterygium, only the worst eye was treated.
All injections were administered by a single investigator in an operating room. Eyes were anesthetized with topical proparacaine hydrochloride drops. A subconjunctival injection of 0.5 mL of bevacizumab (Avastin® 2.5 mg/0.1 mL, F. Hoffmann-La Roche, Basel, Switzerland) was administered at the limbus, adjacent to the pathological blood vessels growing/sprouting into the cornea. Using an eyelid speculum, injections were administered at the slit lamp following application of topical anesthetic eyedrops. After surgery, patients were treated with topical Cilodex® (ciprofloxacyn and dexamethasone, Alcon Laboratories Ltd.) eye drops 4 times daily for 15 days.
As per our protocol, all eyes received a single bevacizumab injection. All eyes were biomicroscopically examined before surgery, 2 and 7 days after surgery, and 1 and 2 months after surgery. Complete opthalmological evaluation was performed for each patient. This included visual acuity determination, applanation tonometry, slit-lamp examination, and anterior segment photography. At each visit, 2 digital corneal photographs were obtained using a digital camera. The size of pterygium was measured horizontally from the limbus to the apex and graded according to the system used by Tan et al. using the slit-lamp microscope(6).
Any complications and adverse events were noted. The same photographer captured images of the anterior segment using the same camera at every follow-up visit. Postinjection complications such as ocular surface toxicity, corneal abrasion, persistent epithelial defect, subconjunctival hemorrhage, and infection were noted, as was any change in the size and vascularity of pterygium.
Statistical analyses were performed using SPSS version 18.0 (SPSS, Chicago, IL). The paired t test was used to compare changes in the size of pterygia, and the Friedman test was used to determine the significance of changes after treatment. Probabilities of less than 5% were considered statistically significant.
RESULTS
The study group comprised 36 eyes of 36 patients with recurrent pterygium. These included 18 males (50%) and 18 females (50%) with a mean age of 58.75 years (SD, 10.98 years). Close to one-third (30.6%) of the patients had recurrent pterygia in both eyes. The left and right eyes were affected in 47.2% and 22.2% patients, respectively. Approximately 44.4% patients were from Goiânia (Brazil), while 55.6% were from other cities. More than half the patients (58.3%) had a family history of pterygium.
According to the results of graph 1 (paired-samples test), there was a significant difference in the size (in mm) of pterygium at different intervals (P<0.05) after the injection of bevacizumab.
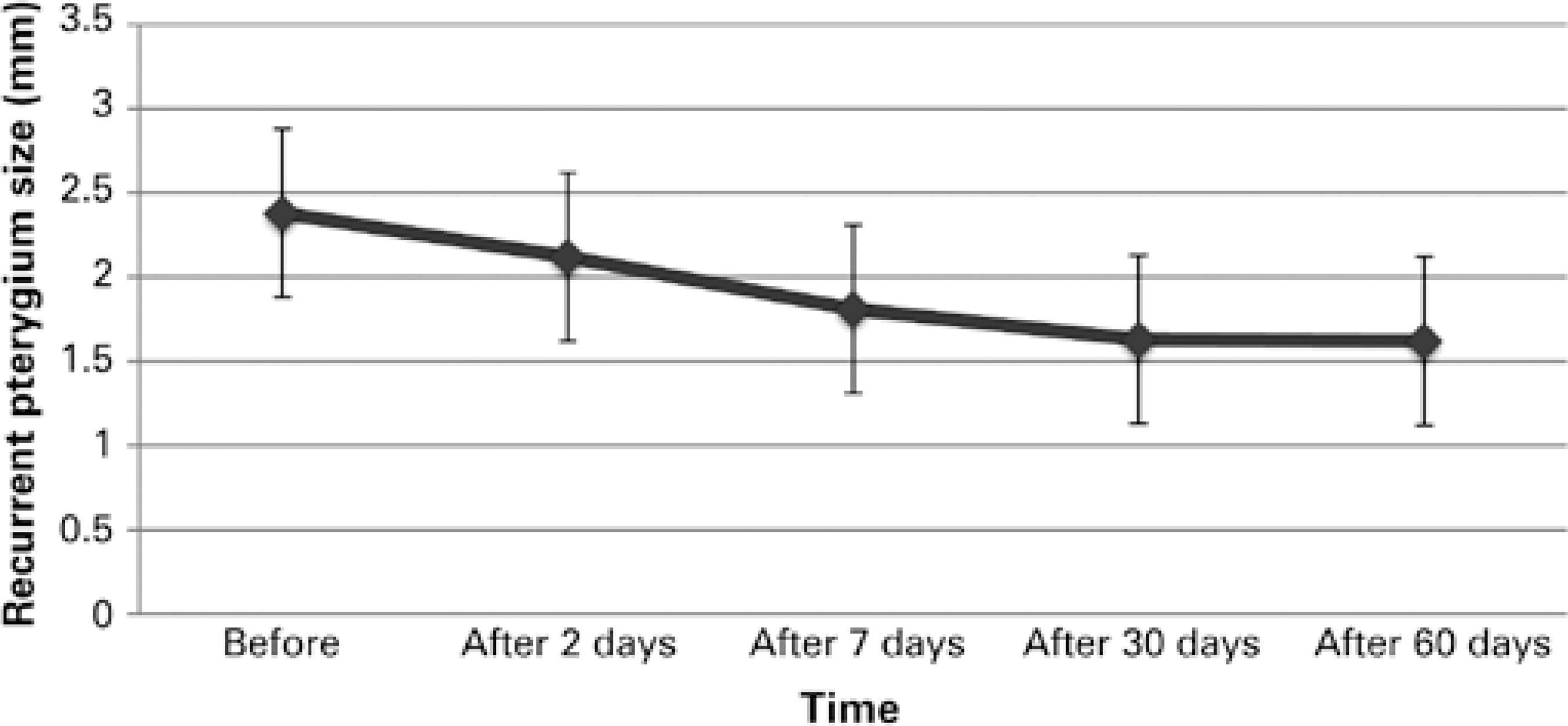
Graph 1 There was a significant change in the size of pterygium at different intervals (P<0.05; paired t-test) after the injection of bevacizumab. The mean pterygium size ranged from 2.37 mm to 1.61 mm. These patients were only the treated ones.
Table 1 represents the distribution of patients according to the classification of Tan et al.(6) before and 60 days after one injection of 0.5 mL of bevacizumab (2.5 mg/0.1 mL). There was a difference in the gradation of pterygium before and after application.
Table 1 Distribution of patients according to the classification of Tan et al.(6) before and 60 days after one application of 0.5 ml of bevacizumab (2.5 mg/0.1 ml)
The mean surface area was decreased. There was a significant difference in the gradation according to the system used by Tan et al.(6) at different intervals (P<0.05), particularly 30 days after injection.
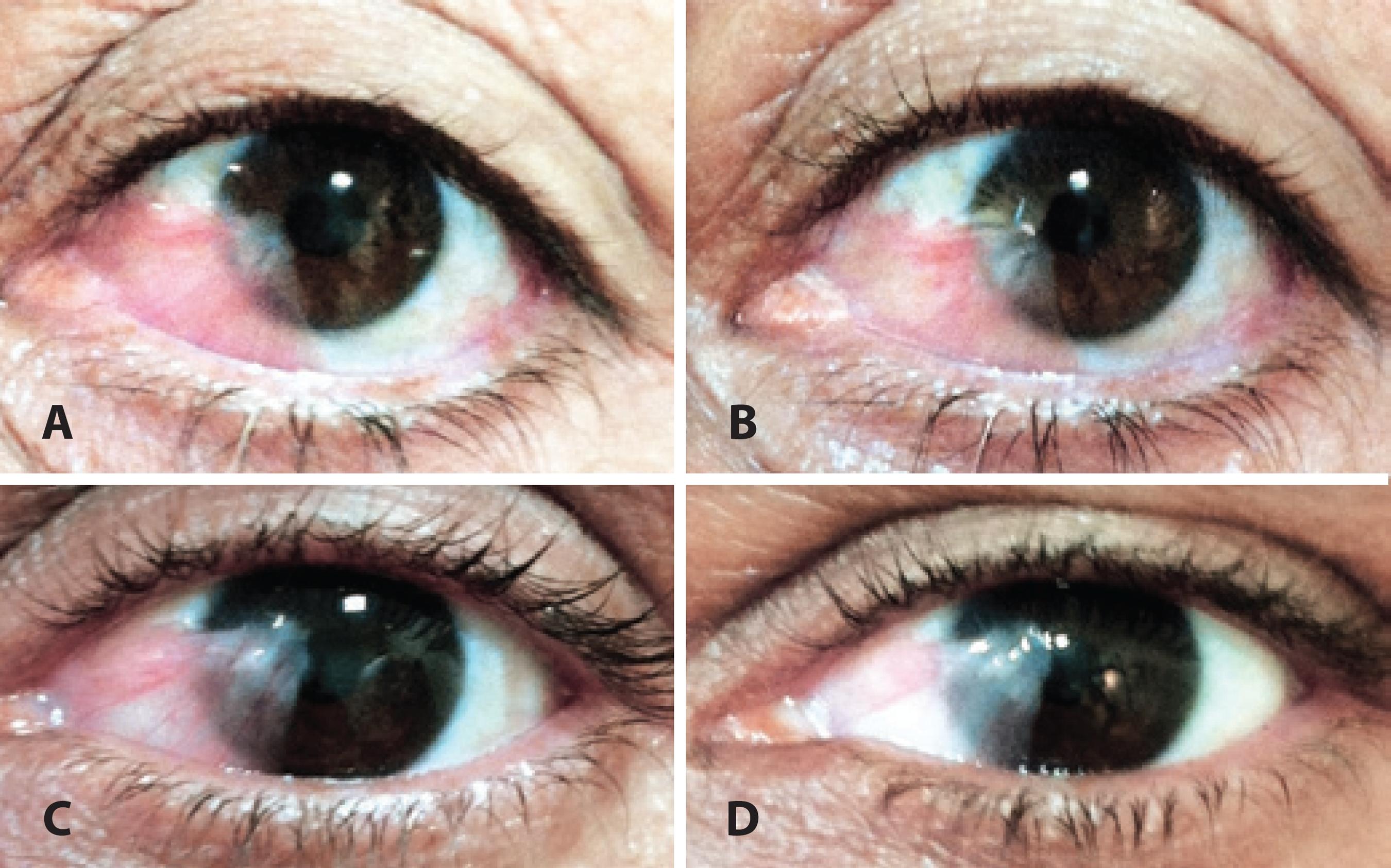
Approximately two-thirds (66.7%) of the patients presented with hyposphagma on the 2nd day after subconjunctival injection; this decreased to 30.6% by day 7 and to 0% at 1 month. Most patients (69.4%) exhibited amelioration of irritative symptoms within 2 days, 88.9% patients after 7 days, and 97.2% after 1 month. The aspect of the recurrent pterygium after injection did not change considerably (Figure 1).
DISCUSSION
In recent years, anti-VEGF agents such as bevacizumab and ranibizumab have been evaluated as an adjunctive therapy for pterygium along with surgical excision or as a nonsurgical therapy(20).
Recent studies have reported successful outcomes after the administration of bevacizumab for the treatment of corneal neovascularization. Erdurmus and Totan(21-22) evaluated the efficacy of subconjunctival bevacizumab injection (2.5 mg/0.1 mL) in 2 patients with corneal neovascularization along with the associated etiologies. One patient had dry eye and exhibited significantly regression of vessels a week after injection, while the other patient had a failed graft and exhibited only minor vessel regression. Awadein(23) described 3 patients with corneal neovascularization after keratoplasty who were treated with a single subconjunctival injection of 2.5 mg bevacizumab. In all patients, the number and caliber of blood vessels decreased after injection. The regression of the corneal new vessels was more marked in patients with smaller and/or fewer blood vessels. However, in patients with old and rejected vascularized grafts, there was little change in the number and caliber of blood vessels(21).
Recently, several studies have been conducted to evaluate the effects of local therapy with bevacizumab on both primary and recurrent pterygium(24-27). Bevacizumab has been used at various doses at various times by different routes of administration. Nonetheless, the results remain limited and controversial, and there is no clear randomized controlled trial that has studied the efficacy of subconjunctival bevacizumab in patients with impending recurrent pterygium(28-30).
A single administration of an arbitrary dose based on the intravitreal preparation of bevacizumab may be inadequate. A single dose of 1.25 mg of intravitreal bevacizumab has been reported to provide complete intravitreal VEGF blockade for a minimum of 4 weeks, with an intravitreal bevacizumab half-life of approximately 3 days(28). However, the conjunctival half-life of the drug may be shorter than the vitreous half-life because of the higher systemic absorption through the abundant conjunctival vessels(24). Repeat injections or higher doses of bevacizumab may be required to achieve better outcomes. Nevertheless, increased or multiple doses may be accompanied by significant side effects.
Our study illustrated that a single subconjunctival dose of bevacizumab dose is partly efficacious in decreasing conjunctival vascularization in the impending recurrent pterygium compared with that at baseline. Nevertheless, the favorable effect was incomplete and temporary, similar to that reported by Leippi et al.(25).
Previous reports suggesting the effective role of subconjunctival bevacizumab were noncomparative case reports(25,28,31). Corneal neovascularization was inhibited or regressed, but not completely eliminated. Delayed recurrence was observed in a study by Fallah et al., who used topical bevacizumab to inhibit the growth of impending recurrent pterygium in 26 eyes, although the pterygia eventually extended onto the cornea(17).
Other factors such as tumor necrosis factor alpha, bFGF, TGF-β, and platelet-derived growth factor (PDGF) have been shown to correlate with the formation and recurrence of pterygium. Immunoreactivity for these growth factors was located in epithelial cells, endothelial cells of vessels, basement membranes of vessels, and the epithelium, fibroblasts, and infiltrating inflammatory cells in pterygium(28,29).
CONCLUSIONS
The limitations of this study include the short duration of follow-up, the lack of a control group, the limited number of patients, and the difficulty in objectively measuring the size of recurrent pterygia before and after injection.
Further studies with larger sample sizes will be required to determine the appropriate dosing schedule, efficacy, and safety profile. In addition, anti-VEGF treatment may have a greater synergistic effect when combined with treatment targeting alternate cytokines and growth factors and should be investigated in future studies. New antiangiogenic therapies will hopefully focus more on facilitating delivery into tissue, increasing the duration of effect while continuing to minimize adverse side effects.
In summary, a single administration of subconjunctival bevacizumab to recurrent pterygium was well tolerated and decreased irritation and vascularization for a short term. The transient effect was likely related to the limited bioavailability of the drug in the setting of continued VEGF expression.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

