

Renato Magalhães Passos1; Angelino Julio Cariello1; Maria Cecília Zorat Yu2; Ana Luisa Höfling-Lima1
DOI: 10.1590/S0004-27492010000400002
ABSTRACT
PURPOSE: To describe the demographic characteristics, associated factors and causative agents of infectious keratitis in the elderly in a tertiary referral center in São Paulo, Brazil. METHODS: A retrospective review of all patients aged 60 years and over with a presumptive diagnosis of infectious keratitis who had material collected for microbiological analysis, between the years 1975 and 2007 (32-year span). RESULTS: From a total of 7,060 age-independent cases of microbial keratitis, 1,545 cases in the elderly were reviewed, which had a mean age of 71.0 ± 7.8 years, ranging from 60 to 101 years. There were 707 males (45.6%) and 838 females (54.3%). Associated factors were: past ocular surgery (25.1%), ocular trauma (7.2%) and contact lens use (3.0%). Bacterioscopy was positive in 40.5% of cases. Culture positivity for any agent was 53.5% (bacteria 47.0%, fungi 6.1%, Acanthamoeba 0.4%). The most frequent bacteria were the gram-positive cocci (mostly coagulase-negative Staphylococci) and gram-negative bacilli (mostly the genera Pseudomonas, Moraxella and Proteus), while the most frequent fungi were the filamentous (mostly the genus Fusarium). CONCLUSIONS: This study represents a large series of microbial keratitis in the elderly in a single referral center. The most important factor associated with this condition in the elderly was past ocular surgery. The most frequent causative agents were bacteria, especially gram-positive cocci and gram-negative bacilli.
Keywords: Keratitis; Eye infections, bacterial; Cornea; Fungi; Blindness; Human; Midlle aged; Review
RESUMO
OBJETIVO: Descrever as características demográficas, fatores associados e agentes etiológicos das ceratites infecciosas em idosos em um centro de referência terciário em São Paulo, Brasil. MÉTODOS: Análise retrospectiva de todos os pacientes a partir de 60 anos com diagnóstico clínico de ceratite infecciosa que tiveram material colhido para análise microbiológica, entre os anos de 1975 e 2007 (intervalo de 32 anos). RESULTADOS: De um total de 7.060 casos de ceratite infecciosa em todas as idades, 1.545 casos em idosos foram revisados. A idade média foi de 71,0 ± 7,8 anos, variando de 60 a 101 anos. Foram 707 homens (45,6%) e 838 mulheres (54,3%). Os principais fatores associados foram: cirurgia ocular prévia (25,1%), trauma ocular (7,2%) e uso de lentes de contato (3,0%). Bacterioscopia foi positiva em 40,5% dos casos. A positividade das culturas para qualquer agente foi de 53,5% (bactérias 47,0%, fungos 6,1%, Acanthamoeba 0,4%). As bactérias mais frequentes foram os cocos gram positivos (principalmente Staphylococcus coagulase negativo) e bacilos gram negativos (principalmente Pseudomonas, Moraxella e Proteus), enquanto os fungos mais frequentes foram os filamentosos (Fusarium). CONCLUSÕES: Este estudo representa até o momento a maior série de casos de ceratite infecciosa em idosos em um centro único. O fator mais associado a esta condição em idosos foi cirurgia ocular prévia. Os agentes etiológicos mais frequentes foram as bactérias, principalmente cocos gram positivos e bacilos gram negativos.
Descritores: Ceratite; Infecções oculares bacterianas; Córnea; Fungos; Cegueira; Humanos; Meia-idade; Revisão
ORIGINAL ARTICLES
Microbial keratitis in the elderly - a 32-year review
Ceratite infecciosa em idosos - revisão de 32 anos
Renato Magalhães PassosI; Angelino Julio CarielloI; Maria Cecília Zorat YuII; Ana Luisa Höfling-LimaI
IPhysician, Ophthalmology Department, Escola Paulista de Medicina, Universidade Federal de São Paulo - UNIFESP - São Paulo (SP), Brazil
IIBiomedical, Ocular Microbiology Lab, Escola Paulista de Medicina, Universidade Federal de São Paulo - UNIFESP - São Paulo (SP), Brazil
ABSTRACT
PURPOSE: To describe the demographic characteristics, associated factors and causative agents of infectious keratitis in the elderly in a tertiary referral center in São Paulo, Brazil.
METHODS: A retrospective review of all patients aged 60 years and over with a presumptive diagnosis of infectious keratitis who had material collected for microbiological analysis, between the years 1975 and 2007 (32-year span).
RESULTS: From a total of 7,060 age-independent cases of microbial keratitis, 1,545 cases in the elderly were reviewed, which had a mean age of 71.0 ± 7.8 years, ranging from 60 to 101 years. There were 707 males (45.6%) and 838 females (54.3%). Associated factors were: past ocular surgery (25.1%), ocular trauma (7.2%) and contact lens use (3.0%). Bacterioscopy was positive in 40.5% of cases. Culture positivity for any agent was 53.5% (bacteria 47.0%, fungi 6.1%, Acanthamoeba 0.4%). The most frequent bacteria were the gram-positive cocci (mostly coagulase-negative Staphylococci) and gram-negative bacilli (mostly the genera Pseudomonas, Moraxella and Proteus), while the most frequent fungi were the filamentous (mostly the genus Fusarium).
CONCLUSIONS: This study represents a large series of microbial keratitis in the elderly in a single referral center. The most important factor associated with this condition in the elderly was past ocular surgery. The most frequent causative agents were bacteria, especially gram-positive cocci and gram-negative bacilli.
Keywords: Keratitis/microbiology; Eye infections, bacterial; Cornea/microbiology; Fungi/isolation & purification; Blindness/prevention & control; Human; Midlle aged; Review
RESUMO
OBJETIVO: Descrever as características demográficas, fatores associados e agentes etiológicos das ceratites infecciosas em idosos em um centro de referência terciário em São Paulo, Brasil.
MÉTODOS: Análise retrospectiva de todos os pacientes a partir de 60 anos com diagnóstico clínico de ceratite infecciosa que tiveram material colhido para análise microbiológica, entre os anos de 1975 e 2007 (intervalo de 32 anos).
RESULTADOS: De um total de 7.060 casos de ceratite infecciosa em todas as idades, 1.545 casos em idosos foram revisados. A idade média foi de 71,0 ± 7,8 anos, variando de 60 a 101 anos. Foram 707 homens (45,6%) e 838 mulheres (54,3%). Os principais fatores associados foram: cirurgia ocular prévia (25,1%), trauma ocular (7,2%) e uso de lentes de contato (3,0%). Bacterioscopia foi positiva em 40,5% dos casos. A positividade das culturas para qualquer agente foi de 53,5% (bactérias 47,0%, fungos 6,1%, Acanthamoeba 0,4%). As bactérias mais frequentes foram os cocos gram positivos (principalmente Staphylococcus coagulase negativo) e bacilos gram negativos (principalmente Pseudomonas, Moraxella e Proteus), enquanto os fungos mais frequentes foram os filamentosos (Fusarium).
CONCLUSÕES: Este estudo representa até o momento a maior série de casos de ceratite infecciosa em idosos em um centro único. O fator mais associado a esta condição em idosos foi cirurgia ocular prévia. Os agentes etiológicos mais frequentes foram as bactérias, principalmente cocos gram positivos e bacilos gram negativos.
Descritores: Ceratite/microbiologia; Infecções oculares bacterianas; Córnea/ microbiologia; Fungos/isolamento & purificação; Cegueira/prevenção & controle; Humanos; Meia-idade; Revisão
INTRODUCTION
Currently, infectious keratitis still represents an important cause of preventable blindness and visual impairment in the world. According to the World Health Organization, the importance of corneal disease as a major cause of blind-ness worldwide remains second only to cataract(1).
Even though the diagnosis of infectious keratitis is usually presumptive and the treatment usually empirical, the laboratorial identification of the causative agent should always be pursued, in order to ensure a more specific treatment, to help guide eventual therapeutic modifications and to minimize any visual impairment due to a wrong or delayed diagnosis(2).
The incidence of different causative agents vary largely according to the geographical region, socioeconomic condition of the population and many individual factors such as contact lens use(3-5), history of trauma(6-7) or past ocular surgery(8-13), presence of comorbidities(14-16), and age of the patient(17), among many others. Several studies have been published regarding the epidemiologic aspects of infectious keratitis on the five continents, both in developed and developing countries(18-24).
Elderly patients are known to have several systemic and ocular peculiarities such as immunologic senescence, comorbidities, alteration of the lids and conjunctival flora, poor lacrimal drainage, fragility of the corneal epithelium, and reduction of corneal sensitivity, among others. These factors are responsible for a specific susceptibility to certain causative agents of microbial keratitis and the establishment of a more aggressive disease(25). Epidemiological studies of keratitis in this age group are scarce in the literature(17,26-29).
Thus, the objective of this study was to describe the epidemiologic and microbiological findings of infectious keratitis in elderly patients in a referral Ophthalmology center in Brazil.
METHODS
A retrospective study was conducted in the Ophthalmology Department of the Federal University of São Paulo (UNIFESP). The records of all patients aged 60 years and older, who were seen between 1975 and 2007 and had a presumptive diagnosis of infectious keratitis and who had ocular material collected for microbiological evaluation were reviewed. The age limit of 60 years was chosen based on WHO criteria for "elderly" in developing countries(30).
The following data were recorded: age, gender, time of onset of the symptoms, history of ocular trauma, previous surgery, contact lens use or topical medication and results of microbiological tests (smears for cytology and bacterioscopy, cultures for bacteria, fungi and protozoa). Data were submitted to descriptive and statistical analysis using Pearson χ2 test and software SPSS® 16.0 for Windows®. Statistical significance was accepted when P<0.05.
In this service, any material collected from corneal ulcers is routinely analyzed by Gram and Giemsa stainings (bacterioscopy and cytology, respectively) and incubated in the following media: blood agar, chocolate agar, Sabouraud agar (solid), brain heart infusion (BHI) and thioglycolate (liquid). When there is suspicion of Acanthamoeba infection, samples are placed in E. coli-enriched solid medium.
This study was approved by the local ethics committee, project number 1719/07.
RESULTS
From July, 1975 to September, 2007, there were 16,573 patients whose samples were sent to the Laboratory of Ocular Microbiology of UNIFESP for analysis, including corneal and conjunctival scrapings, aqueous and vitreous samples, secretions, contact lenses and other objects, among many others. Of these, 7,060 (42.6%) were from patients with a presumptive diagnosis of infectious keratitis and 1,545 (9.3%) were elderly patients with suspected keratitis.
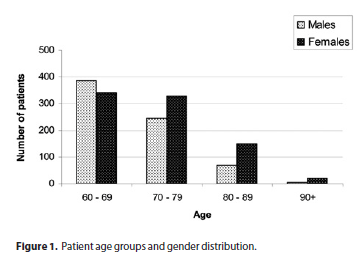
The distribution according to age and gender is shown in figure 1. There were 838 females (54.2%) and 707 males (45.8%) with a female:male ratio of 1.18. Age varied from 60 to 101 years, with a mean age of 71.0 ± 7.8 years. The most prevalent age group was 60-69 years (46.9%) followed by 70-79 years (36.9%), 80-89 years (14.4%) and > 90 years (1.6%).
The number of days between the onset of symptoms and the first laboratory analysis was also assessed. The mean was 17.6 ± 22.0 days, ranging from 1 day to several months, with a median of 8 days.
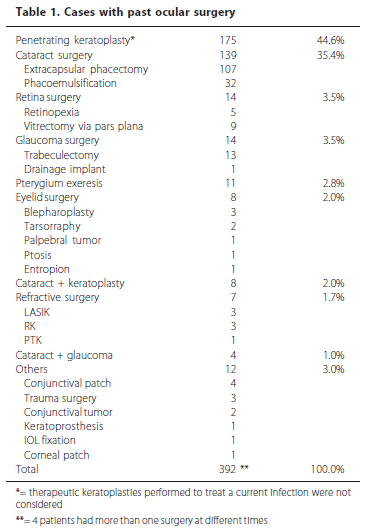
History of past ocular surgery was present in 388 patients (25.1%). Table 1 shows the distribution by type of surgery. The time interval between the previous surgery and the episode of keratitis was divided into three categories: up to 15 days (early postoperative period), 16 to 60 days (intermediate period) and over 60 days (late postoperative period). The distribution was 7.9%, 25.9% and 66.2% of patients with past ocular surgery, respectively.
History of ocular trauma was present in 112 patients (7.2%), including chemical burns. Of these, 49.1% had history of trauma from plant materials. Contact lens wear was present in 47 patients (3.0%).
Previous use of topical medication was present in 552 patients (35.7%). Of these, 424 (27.4%) were using antibiotic drops (alone or in combination with other drugs) and 36 (2.3%) were using antifungal drops. However, we do not have precise information regarding whether the medication had been suspended before the corneal scraping.
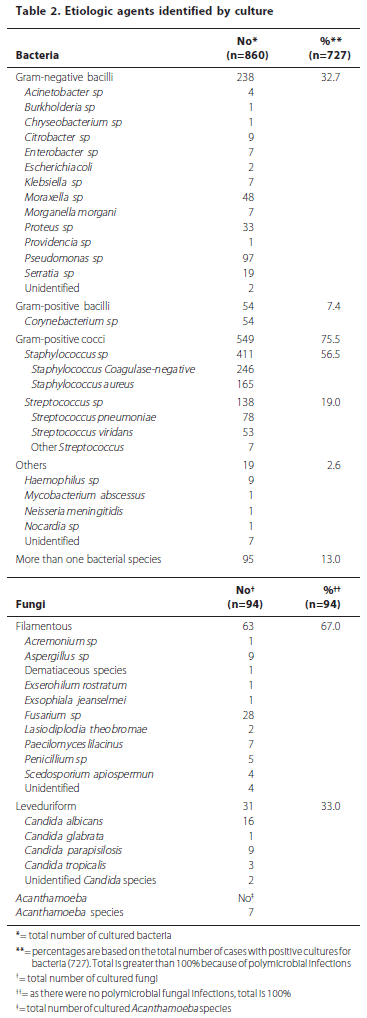
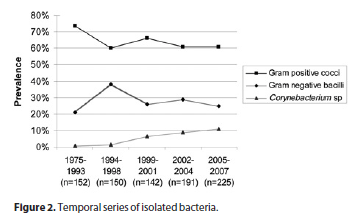
Smears were positive for microorganisms in 554 cases (35.8%). Cultures for bacteria were positive in 727 cases (47.0%). The total number of positive bacterial cultures was 860, due to polymicrobial infections. Cultures for fungi were positive in 94 cases (6.1%). Cultures for Acanthamoeba were performed in 57 patients. Of these, 7 had a positive culture (12.3% of suspected cases and 0.4% of total). Total culture positivity for any agent was 53.5%. Despite the diagnostic efforts, the remaining 46.5% of cases did not show any identifiable causative agent. Details from the microbiological profile are described in table 2. The temporal analysis, dividing the 32-year period into 5 sub-periods with an equivalent number of patients, showed an increase in the frequency of gram-negative bacilli and a decrease in the frequency of gram-positive cocci from 1975-93 to 1994-98, followed by a relatively constant proportion of each bacterial type from 1999 onwards. The frequency of Corynebacterium sp increased from 0.7% to 11.1% in a gradual fashion over the whole time span of the study. Details of this analysis are found in figure 2.
A comparison between this study and four other recent studies on infectious keratitis in the elderly(17,26,28-29) was also performed, which is detailed in table 3. The largest sample size before this study with 1,545 patients was from Butler et al.(26), with 190 patients. The prevalence of three important risk factors associated with microbial keratitis in the elderly, namely past ocular surgery, trauma and contact lens use, was somewhat conflicting among the studies. Culture positivity was only similar to that reported by van der Meulen et al.(29) (53.5% vs 54.5%) while smaller than in other studies. The proportions of isolated bacteria (47% to 64%) and fungi (0% to 32.7%) were also variable among the studies while that of Acanthamoeba keratitis was relatively constant (maximum of 1%).
DISCUSSION
Although this study represents so far the largest series of infectious (non-viral) keratitis in the elderly at a single referral center, it has some limitations: the ones intrinsic to the retrospective design, the absence of rigid inclusion/exclusion criteria (all cases that underwent corneal scraping with the presumptive diagnosis of infectious keratitis were included in the database) and incomplete data (especially in the early years). Still, its importance relies on the fact that it represents an experience of 32 years in the management of infectious keratitis in the elderly at a single referral center.
DEMOGRAPHIC FEATURES
Unlike other studies on keratitis in the elderly(17,26,28-29), females made up the majority in our sample. Probably due to the greater number of subjects in this study, this female preponderance reflects a general demographic tendency in the elderly(31). Figure 1 illustrates that while males were the majority in the age group of 60 to 69 years, females were the majority in all age groups above 69 years, probably because of the higher life expectancy of the latter(31). There does not seem to be any increased susceptibility to microbial keratitis in either gender.
PREDISPOSING FACTORS
The considerable prevalence of past ocular surgery (25.1%) in this study is in accordance with previous studies on the elderly(17,26,28). The prevalence of this predisposing factor in recent age-independent series of infectious keratitis varied from 11 to 17%(32-35), suggesting that such variable is more frequent in the elderly.
Penetrating keratoplasty and cataract surgery together occurred in 80% of all cases with previous ocular surgery. Over half (66.2%) of the patients had undergone surgery more than two months before the onset of keratitis and only 7.9% fifteen days before, suggesting that chronic changes induced by the surgery could be more relevant risk factors than early postoperative conditions. Moreover, the regular use of antibiotic prophylaxis by recently operated patients certainly reduces the incidence of infectious keratitis in such cases(36-37). On the other hand, these numbers may be partially explained by the accumulated number of cases with past ocular surgery over time that might not have had any relation at all with keratitis at the time of diagnosis. In fact, when matching patients with any previous surgery versus culture-positive cases (for bacteria or fungi), there was no significant statistical correlation, but a trend (OR=1.21, P=0.097). However, when the analysis was performed considering only keratoplasty cases, the correlation was more evident (OR=1.36, P=0.05). Penetrating keratoplasty may be considered a potentially hazardous procedure on a long-term basis due to several factors that could lead to infectious keratitis: chronic surface alterations such as irregular tear film distribution, corneal hypoesthesia and punctate keratitis, graft failure with secondary epithelial defects, and loose and torn sutures, among others(8-9). The high prevalence of cataract surgery is probably due to it being the most performed ocular surgery during the study period, and not due to a specific risk factor induced by the surgery itself. In fact, there was no statistical correlation between cataract-operated patients and culture positivity (P=0.45).
The prevalence of ocular trauma varied considerably among the studies on the elderly, from 3.7 to 43.6%(17,26,28-29), but was relatively constant in the age-independent series, ranging from 16 to 26.6%(32-35). Contact lens wear was found in only 3.0% of cases, like the already mentioned studies with the exception of van der Meulen et al.(29), which found 13.0%. In the age-independent studies already mentioned, the rate of contact lens wear varied from 3.2 to 44.3%. As discussed by Butler et al.(26), the elderly differ from younger age groups in types of predisposing factors. Contact lens wear and ocular trauma are much more frequent in adults, while previous ocular surgery and pre-existing diseases (ocular or systemic) play a substantial role in the elderly.
Considering the more recognized predisposing factors for infectious keratitis(33) - ocular surgery, trauma and contact lens wear - around one-third of patients (35.3%) in our study presented with either of these conditions. Many other systemic or ocular diseases may also predispose to infectious keratitis, though this information was underestimated in our series due to incomplete data. Analyzing the time of symptoms before the patient had the first laboratory analysis, 50% had up to 8 days of history, reflecting the acute course of most cases of microbial keratitis, especially bacterial. Only 40.3% of patients came between 8 and 30 days after onset of symptoms and 12.0% after 30 days, which is considered extremely late and may impair irreversibly the possibilities of treatment. Besides, some patients only appeared at the referral service for a more complex diagnostic approach after having gone through other doctors and sometimes were already under antibiotic treatment.
MICROBIOLOGY
Culture positivity in this series was lower compared to three other studies on the elderly(17,26,28) and similar to one study(29). This may be due to the greater number of cases in this study or an underestimation of the number of previously treated patients at the time of corneal scraping. It was difficult to determine if the previous use of antimicrobial medication had any impact on culture positivity, because we did not have the information on whether or not medication had been suspended before the scrapings, and for how many days.
Among the bacteria isolated, Gram-positive cocci continue to be the most frequent agent, with a similar rate compared to the other studies in both elderly and age-independent series(17,20,26,28-29,32-35,38-43). The incidence of Gram-negative bacilli was a little higher, which could be explained by geographical variation in the microbial flora of our patients(2,14-15,44). The temporal analysis showed from 1975-93 to 1994-98 an increase in the frequency of gram-negative bacilli and a decrease in the frequency of gram-positive cocci, which from 1999 onwards remained stable. There does not seem to be a rational explanation for this fact, even though the first period of 1975 to 1993 contributed little to the whole sample due to administrative issues and insufficient notification at the time. The increasing incidence of Corynebacterium sp over the years was somewhat surprising and was probably due to improvements in microbiological techniques for the isolation of said bacteria.
The percentage of fungal keratitis was the most conflicting between the studies, ranging from 0% to 32.7%(17,26,28-29). Parmar et al.(17) showed the highest prevalence of fungal infection in both the elderly (32.7%) and the non-elderly control group (39.9%). In a large series of age-independent infectious keratitis from the same group and hospital(24), the prevalence of fungal infection was 56.1%. This was probably because of the great proportion of inhabitants from rural areas seen on that service. In urban centers, as in our study and the Australian and the Dutch studies(26,29), the prevalence of fungal agents seems to be around 0 to 7%. The prevalence of keratitis by Acanthamoeba seems to follow a constant rate in this age group, up to 1%(17,26,28-29).
CONCLUSIONS
Females comprised the majority in our series.
Among the predisposing factors, previous ocular surgery seemed to be the most important in the elderly (pre-existing ocular diseases were not evaluated in this study), with penetrating keratoplasty being the most associated with culture positivity. Ocular trauma and contact lens wear were not as important in the elderly as in the younger adult population.
The most prevalent causative agents in this series were the gram-positive cocci (Staphylococcus and Streptococcus species) and the gram-negative bacilli (especially Pseudomonas, Moraxella and Proteus species). The prevalence of fungal and Acanthamoeba infections in the elderly seemed to follow the trends in urban centers. Despite the efforts, in almost half of the cases it was not possible to identify the causative agent of keratitis, thus emphasizing the importance of clinical judgment and empirical treatment in this threatening condition.
Prospectively, the increased availability of molecular techniques such as polymerase chain reaction (PCR) for the diagnosis of ocular infections will allow a better diagnosis and management of previously culture-negative cases, due to the high accuracy of these tests.
REFERENCES
1. Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79(3):214-21.
2. Solari HP, Sousa LB de, Freitas D de, Yu MCZ, Hogling-Lima AL. Características laboratoriais das ceratites e conjuntivites causadas por Streptococcus sp. Arq Bras Oftalmol. 2004;67(5):781-4.
3. Morgan PB, Efron N, Hill EA, Raynor MK, Whiting MA, Tullo AB. Incidence of keratitis of varying severity among contact lens wearers. Br J Ophthalmol. 2005;89(4):430-6.
4. Lipener C, Ribeiro AL. Bilateral Pseudomonas corneal ulcer in a disposable contact lens wearer. CLAO J. 1999;25(2):123-4.
5. Lipener C, Nagoya FR, Zamboni FJ, Lewinski R, Kwitko S, Uras R. Bacterial contamination in soft contact lens wearers. CLAO J. 1995;21(2):122-4.
6. Stefan C, Nenciu A. Post-traumatic bacterial keratitis -a microbiological prospective clinical study. Oftalmologia. 2006;50(3):118-22.
7. Upadhyay MP, Karmacharya PC, Koirala S, Shah DN, Shakya S, Shrestha JK, et al. The Bhaktapur eye study: ocular trauma and antibiotic prophylaxis for the prevention of corneal ulceration in Nepal. Br J Ophthalmol. 2001;85(4):388-92.
8. Wagoner MD, Al-Swailem SA, Sutphin JE, Zimmerman MB. Bacterial keratitis after penetrating keratoplasty: incidence, microbiological profile, graft survival, and visual outcome. Ophthalmology. 2007;114(6):1073-9.
9. Sharma N, Gupta V, Vanathi M, Agarwal T, Vajpayee RB, Satpathy G. Microbial keratitis following lamellar keratoplasty. Cornea. 2004;23(5):472-8.
10. Charteris DG, Batterbury M, Armstrong M, Tullo AB. Suppurative keratitis caused by Streptococcus pneumoniae after cataract surgery. Br J Ophthalmol. 1994;78(11): 847-9.
11. Kehdi EE, Watson SL, Francis IC, Chong R, Bank A, Coroneo MT, et al. Spectrum of clear corneal incision cataract wound infection. J Cataract Refract Surg. 2005;31(9):1702-6.
12. Leal F, Höfling-Lima AL, de Freitas D, Campos M. [Laboratory analysis of infectious keratitis in patients following refractive surgery.]. Arq Bras Oftalmol. 2005;68(3):353-6. Portuguese.
13. Vieira AC, Pereira T, de Freitas D. Late-onset infections after LASIK. J Refract Surg. 2008;24(4):411-3.
14. Martins EN, Alvarenga LS, Höfling-Lima AL, Freitas D, Zorat-Yu MC, Farah ME, et al. Aerobic bacterial conjunctival flora in diabetic patients. Cornea. 2004;23(2):136-42.
15. Andrade AJ, Höfling-Lima AL, Yu MC, Godoy P, Gompertz OF, Bonfim S de S, et al. [Study of mycobiota in the healthy conjunctiva of diabetics who reside in the urban area of the city of São Paulo, Brazil]. Arq Bras Oftalmol. 2006; 69(1):75-83. Portuguese.
16. Höfling-Lima AL, Forseto A, Duprat JP, Andrade A, Souza LB, Godoy P, et al. [Laboratory study of the mycotic infectious eye diseases and factors associated with keratitis]. Arq Bras Oftalmol. 2005;68(1):21-7. Portuguese.
17. Parmar P, Salman A, Kalavathy CM, Kaliamurthy J, Thomas PA, Jesudasan CA. Microbial keratitis at extremes of age. Cornea. 2006;25(2):153-8.
18. Bharathi MJ, Ramakrishnan R, Vasu S, Meenakshi R, Palaniappan R. Epidemiological characteristics and laboratory diagnosis of fungal keratitis. A three-year study. Indian J Ophthalmol. 2003;51(4):315-21.
19. Chowdhary A, Singh K. Spectrum of fungal keratitis in North India. Cornea. 2005; 24(1):8-15.
20. Gonzales CA, Srinivasan M, Whitcher JP, Smolin G. Incidence of corneal ulceration in Madurai district, South India. Ophthalmic Epidemiol. 1996;3(3):159-66. Comment in: Ophthalmic Epidemiol. 1998;5(1):3-6.
21. Gopinathan U, Garg P, Fernandes M, Sharma S, Athmanathan S, Rao GN. The epidemiological features and laboratory results of fungal keratitis: a 10-year review at a referral eye care center in South India. Cornea. 2002;21(6):555-9. Review.
22. Sharma S, Gopalakrishnan S, Aasuri MK, Garg P, Rao GN. Trends in contact lens-associated microbial keratitis in Southern India. Ophthalmology. 2003; 110(1):138-43.
23. Srinivasan M, Gonzales CA, George C, Cevallos V, Mascarenhas JM, Asokan B, et al. Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, South India. Br J Ophthalmol. 1997;81(11):965-71.
24. Leck AK, Thomas PA, Hagan M, Kaliamurthy J, Ackuaku E, John M, et al. Aetiology of suppurative corneal ulcers in Ghana and south India, and epidemiology of fungal keratitis. Br J Ophthalmol. 2002;86(11):1211-5.
25. Russ HH, Kara-José N. Processos degenerativos da conjuntiva, córnea e esclera. In: Kara-José N, Almeida G, editores. Senilidade ocular. São Paulo: Roca; 2001. p.85-98.
26. Butler TK, Spencer NA, Chan CC, Singh Gilhotra J, McClellan K. Infective keratitis in older patients: a 4 year review, 1998-2002. Br J Ophthalmol. 2005; 89(5):591-6.
27. Ormerod LD. Causes and management of bacterial keratitis in the elderly. Can J Ophthalmol. 1989;24(3):112-6.
28. Kunimoto DY, Sharma S, Garg P, Gopinathan U, Miller D, Rao GN. Corneal ulceration in the elderly in Hyderabad, South India. Br J Ophthalmol. 2000; 84(1):54-9.
29. van der Meulen IJ, van Rooij J, Nieuwendaal CP, Van Cleijnenbreugel H, Geerards AJ, Remeijer L. Age-related risk factors, culture outcomes, and prognosis in patients admitted with infectious keratitis to two Dutch tertiary referral centers. Cornea. 2008; 27(5):539-44.
30. World Health Organization. Definition of an older or elderly person: proposed working definition of an older person in Africa for the mos project [Internet]. Geneva: WHO. [cited 2010 Jul 2]. Available from: http://www.who.int/healthinfo/survey/ageingdefnolder/en/index.html
31. World Health Organization. WHO Statistical Information System (WHOSIS). Geneva: WHO; 2006. [cited 2010 Jul 2]. Available from: http://www.who.int/whosis/en/index.html
32. Fong CF, Tseng CH, Hu FR, Wang IJ, Chen WL, Hou YC. Clinical characteristics of microbial keratitis in a university hospital in Taiwan. Am J Ophthalmol. 2004; 137(2):329-36.
33. Green M, Apel A, Stapleton F. Risk factors and causative organisms in microbial keratitis. Cornea. 2008;27(1):22-7.
34. Sirikul T, Prabriputaloong T, Smathivat A, Chuck RS, Vongthongsri A. Predisposing factors and etiologic diagnosis of ulcerative keratitis. Cornea. 2008;27(3):283-7.
35. Yilmaz S, Ozturk I, Maden A. Microbial keratitis in West Anatolia, Turkey: a retrospective review. Int Ophthalmol. 2007;27(4):261-8.
36. Baum J, Barza M. The evolution of antibiotic therapy for bacterial conjunctivitis and keratitis: 1970-2000. Cornea. 2000;19(5):659-72. Review.
37. Mather R, Karenchak LM, Romanowski EG, Kowalski RP. Fourth generation fluoroquinolones: new weapons in the arsenal of ophthalmic antibiotics. Am J Ophthalmol. 2002;133(4):463-6.
38. Dunlop AA, Wright ED, Howlader SA, Nazrul I, Husain R, McClellan K, Billson FA. Suppurative corneal ulceration in Bangladesh. A study of 142 cases examining the microbiological diagnosis, clinical and epidemiological features of bacterial and fungal keratitis. Aust N Z J Ophthalmol. 1994;22(2):105-10.
39. Hagan M, Wright E, Newman M, Dolin P, Johnson G. Causes of suppurative keratitis in Ghana. Br J Ophthalmol. 1995;79(11):1024-8.
40. Hall RC, McKellar MJ. Bacterial keratitis in Christchurch, New Zealand, 1997-2001. Clin Experiment Ophthalmol. 2004;32(5):478-81.
41. Keay L, Edwards K, Naduvilath T, Taylor HR, Snibson GR, Forde K, et al. Microbial keratitis predisposing factors and morbidity. Ophthalmology. 2006;113(1):109-16. Comment in: Ophthalmology. 2006;113(11):2115-6; author reply 2116.
42. Laspina F, Samudio M, Cibils D, Ta CN, Fariña N, Sanabria R, et al. Epidemiological characteristics of microbiological results on patients with infectious corneal ulcers: a 13-year survey in Paraguay. Graefes Arch Clin Exp Ophthalmol. 2004;242(3):204-9.
43. Poole TR, Hunter DL, Maliwa EM, Ramsay AR. Aetiology of microbial keratitis in northern Tanzania. Br J Ophthalmol. 2002;86(8):941-2.
44. Moeller CT, Branco BC, Yu MC, Farah ME, Santos MA, Höfling-Lima AL. Evaluation of normal ocular bacterial flora with two different culture media. Can J Ophthalmol. 2005;40(4):448-53.
 Correspondence address:
Correspondence address:
Renato Magalhães Passos
Department of Ophthalmology, Federal University of São Paulo - UNIFESP
R. Botucatu, 820
CEP 04023-062. São Paulo (SP), Brazil
E-mail: [email protected]
Recebido para publicação em 19.01.2010
Aprovação em 01.07.2010
Financial support and proprietary interests: none
Study carried out at Ophthalmology Department, Escola Paulista de Medicina, Universidade Federal de São Paulo - UNIFESP - São Paulo (SP), Brazil.