

Rogil José de Almeida Torres1; Maurício Maia2; Dalton Bertolin Précoma3; Lucia Noronha4; Andréa Luchini5; Leonardo Brandão Précoma6; Greyce Kelly de Souza6; Cristina Muccioli7
DOI: 10.1590/S0004-27492009000600010
ABSTRACT
PURPOSE: The aim of this study is to demonstrate the early changes of the sensory retina induced by hypercholesterolemia in an experimental model. METHODS: New Zealand rabbits were divided into two groups: CG (Control Group) was fed a normal diet for 6 weeks. G1 was initially fed a 1% cholesterol diet for two weeks and from the 14th day on a 0.5% cholesterol diet until the 42nd day. The eyes underwent an immunohistochemical analysis with monoclonal antibodies anti-calretinin and anti-glial fibrillary acidic protein (GFAP). RESULTS: G1 cells and cell elements presented significant immunoreactivity to anti-calretinin. No immunoreactivity to anti-glial fibrillary acidic protein was observed in both groups. CONCLUSION: This study has shown that a hypercholesterolemic diet may induce early changes in the sensory retina in rabbits. The anti-calretinin monoclonal antibody was able to reveal calcium accumulation inside the nerve cells.
Keywords: Retina; Models, animal; Calcium-binding proteins; Cholesterol; Anoxia; Immunohistochemistry; Rabbits
RESUMO
OBJETIVO: O objetivo deste estudo é demonstrar experimentalmente as alterações precoces da retina sensorial induzidas pela hipercolesterolemia. MÉTODOS: Coelhos New Zealand foram organizados em dois grupos: GC (grupo controle), composto por 6 coelhos (6 olhos), recebeu dieta normal por 6 semanas; G1, composto por 12 coelhos (12 olhos), tratado previamente com ração colesterol a 1% (Sigma-Aldrich) por 2 semanas e a partir do 14º dia com ração colesterol a 0,5% (Sigma-Aldrich). Os olhos foram submetidos à análise imunohistoquímica com os anticorpos monoclonais anticalretinina e anti-glial fibrillary acidic protein (GFAP). RESULTADOS: G1 apresentou maior número de células e elementos celulares imunoreativos a anticalretinina que o GC, com relevância estatística. GFAP foi negativo em ambos os grupos. CONCLUSÃO: Este estudo demonstrou que a dieta hipercolesterolêmica pode induzir alterações precoces na retina sensorial em coelhos. O anticorpo monoclonal anticalretinina foi capaz de revelar o acúmulo de cálcio dentro das células neuronais retiniana.
Descritores: Retina; Modelos animais; Proteínas de ligação do cálcio; Colesterol; Anoxia; Imuno-histoquímica; Coelhos
ORIGINAL ARTICLE
Evaluation of early abnormalities of the sensory retina in a hypercholesterolemia experimental model: an immunohistochemical study
Avaliação das anormalidades precoces da retina sensorial em modelo experimental de hipercolesterolemia: estudo imunohistoquímico
Rogil José de Almeida TorresI; Maurício MaiaII; Dalton Bertolin PrécomaIII; Lucia NoronhaIV; Andréa LuchiniV; Leonardo Brandão PrécomaVI; Greyce Kelly de SouzaVI; Cristina MuccioliVII
IDoctoral student at the Ophthalmology Department, Universidade Federal de São Paulo - UNIFESP - São Paulo (SP) - Brazil. Physician in charge of the Medical Residence at the Hospital Angelina Caron - Curitiba (PR) - Brazil
IIGraduate Program Advisor at the Ophthalmology Department, UNIFESP - São Paulo - SP. Ophthalmologist, Director of the Vitreoretinal Surgery Service, Hospital de Olhos Oeste Paulista - Assis (SP) - Brazil
IIIAssistant Professor at the Cardiology Department, Pontifícia Universidade Católica do Paraná - PUCPR - Curitiba (PR) - Brazil
IVAssistant Professor at the Pathology Department, PUCPR - Curitiba (PR) - Brazil
VOphthalmologist at the Centro Oftalmológico de Curitiba (PR) - Brazil
VIMedical Student at the PUCPR - Curitiba (PR) - Brazil
VIIAssistant Professor at the Ophthalmology Department, UNIFESP - São Paulo (SP) - Brazil
ABSTRACT
PURPOSE: The aim of this study is to demonstrate the early changes of the sensory retina induced by hypercholesterolemia in an experimental model.
METHODS: New Zealand rabbits were divided into two groups: CG (Control Group) was fed a normal diet for 6 weeks. G1 was initially fed a 1% cholesterol diet for two weeks and from the 14th day on a 0.5% cholesterol diet until the 42nd day. The eyes underwent an immunohistochemical analysis with monoclonal antibodies anti-calretinin and anti-glial fibrillary acidic protein (GFAP).
RESULTS: G1 cells and cell elements presented significant immunoreactivity to anti-calretinin. No immunoreactivity to anti-glial fibrillary acidic protein was observed in both groups.
CONCLUSION: This study has shown that a hypercholesterolemic diet may induce early changes in the sensory retina in rabbits. The anti-calretinin monoclonal antibody was able to reveal calcium accumulation inside the nerve cells.
Keywords: Retina; Models, animal; Calcium-binding proteins; Cholesterol; Anoxia; Immunohistochemistry; Rabbits
RESUMO
OBJETIVO: O objetivo deste estudo é demonstrar experimentalmente as alterações precoces da retina sensorial induzidas pela hipercolesterolemia.
MÉTODOS: Coelhos New Zealand foram organizados em dois grupos: GC (grupo controle), composto por 6 coelhos (6 olhos), recebeu dieta normal por 6 semanas; G1, composto por 12 coelhos (12 olhos), tratado previamente com ração colesterol a 1% (Sigma-Aldrich) por 2 semanas e a partir do 14º dia com ração colesterol a 0,5% (Sigma-Aldrich). Os olhos foram submetidos à análise imunohistoquímica com os anticorpos monoclonais anticalretinina e anti-glial fibrillary acidic protein (GFAP).
RESULTADOS: G1 apresentou maior número de células e elementos celulares imunoreativos a anticalretinina que o GC, com relevância estatística. GFAP foi negativo em ambos os grupos.
CONCLUSÃO: Este estudo demonstrou que a dieta hipercolesterolêmica pode induzir alterações precoces na retina sensorial em coelhos. O anticorpo monoclonal anticalretinina foi capaz de revelar o acúmulo de cálcio dentro das células neuronais retiniana.
Descritores: Retina; Modelos animais; Proteínas de ligação do cálcio; Colesterol; Anoxia; Imuno-histoquímica; Coelhos
INTRODUCTION
Experimental studies have demonstrated that a cholesterol-enriched diet induces changes in endothelial cells of the intraretinal and epiretinal capillaries, an increase in lipids in the outer retina and changes in the Bruch's membrane(1-5). These changes induce chronic ischemia of the retinal tissue(3,6). Hypoxia induces an increase in the excitatory glutamate neurotransmitter which acts upon the N-metil-D-aspartate (NMDA) receptors located in the neuron plasma membrane and neuron glia(7). Hyperstimulation of NMDA glutamatergic receptors induces an increase in intracellular calcium to toxic levels. The increase of intracellular Ca (2+) suggests neuronal deterioration which may cause its death. It has been shown that the excessive calcium influx stimulates nitric oxide synthase (NOS), which is responsible for the formation of nitric oxide (ON)(8). It has also been demonstrated that hypercholesterolemia increases NOS-2 expression in the retina, inducing lipid peroxidation which causes oxidative tissue damage(9). Calcium influx into retinal cells can also be accounted for by the interference in the production of essential polyunsaturated fatty acids (PUFAs) induced by hypercholesterolemia(10). In some species, diets deficient in omega-3 or PUFAs, have been responsible for the decrease in docosahexaenoic acid (DHA) in the brain and retina, which can cause neural and visual damage(11). Such conditions may cause or aggravate AMD.
The objective of this experimental study is to demonstrate that a cholesterol-enriched diet induces early abnormalities in the neurosensory retina. The authors performed immunohistochemical analysis with the anti-calretinin antibody, aiming to detect any presence of intracellular calcium(12), and thus, detect evidence of the start of neural retina deterioration. To complement the retina analysis, GFAP antibody (anti-glial fibrillary acidic protein) were also used to demonstrate the possible formation of post-mortem neural glial tissue.
METHODS
This study was approved by the Investigational Review Board of Animal Experimentation of Angelina Caron Hospital and the Federal University of São Paulo. In accordance with the Helsinki Declaration and with the ARVO Guidelines for the Use of Animals in Ophthalmic and Vision Research.
In this study 18 white male New Zeland rabbits (Oryctolagus cunicullus) were used. The average weight and age of the rabbits were 2,500 g and 4 months old, respectively. These animals underwent an adaptation period of 7-10 days before the beginning of the experiment. They were kept in a bioterium (macroenvironment) on 12/12 hour light cycles, with air changes and room temperature controlled between 19 to 23ºC. The animals were housed in metal cages which were cleaned daily (microenvironment). During the experiment they received water and were fed a standard diet for rabbits Nuvital® (Nuvital, Colombo, Brazil) ad libitum.
Experiment Outline
The rabbits were divided into two groups:
A) Control Group (CG), consisted of 6 rabbits which were fed a standard rabbit diet for 6 weeks.
B) Group 1 (G1), consisted of 12 rabbits that were initially fed using 1% cholesterol diet Sigma-Aldricht® (Sigma-Aldrich, St Louis, USA) for two weeks, followed by a 0.5% cholesterol diet Sigma-Aldrich® (Sigma-Aldrich, St Louis, USA) for 4 weeks.
The diet was prepared every 14 days, with a 48-hour waiting period before being administered to the animals. The daily fed for each animal was 600 grams(13).
Blood serum analysis
The rabbits underwent the following examinations: seric dosages of total cholesterol, triglycerides, HDL cholesterol and glycemia at the beginning of the experiment, on the 14th day and on the 42nd day.
Tissue preparation
The animals were euthanized on the 42nd day by a 5 ml endovenous pentobarbital injection and the eyes were immediately fixed in 4% paraformaldehyde (Merck, Darmstadt, Germany), at 4º C, in 0.1 M phosphate/ pH 7.4 for 4 hours. Only one eye of each animal was used for this study. After fixation, the samples were evaluated macroscopically: a coronal section at the level of the optic nerve was performed, dividing the ocular globe into two equal halves (upper and lower). The lower half was stored for future studies. Conversely, the upper half underwent dehydration, diaphanization and paraffin embedding with a Leica® TP 1020 - Automatic Tissue Processor (Leica, Wetzlar,Germany). A Leica® EG1160 paraffin embedding device was used to produce paraffin blocks. To obtain 5 μ-thick histological sections, the Leica® RM2145 Microtome was used (Leica, Wetzlar, Germany). The sections were placed on glass slides smeared with albumin, stained with Hematoxiline - Eosine (HE) and mounted with 24 X 900 mm coverslips, using Entellan, Merck® (Merck, Darmstadt, Germany).
Preparation and immunohistochemical analysis
The histological slices were submitted to blockage of endogenous peroxidase. They were then washed in deionized water and incubated in wet chamber at 95º C for 20 minutes for antigen recovery. After this phase, a new blockage of endogenous peroxidase was performed. The slices were covered with primary mouse monoclonal antibody (Dako® calretinin, clone DAK Calret 1, at dilution 1:200 or GFAP by Dako®, clone GF2 at 1:300 dilution) and incubated for 24 hours at room temperature. They were then incubated with secondary antibody, Polymer-HRP Anti-Mouse Envision® System (DakoCytomation, Inc., Carpinteria, CA) for 30 minutes. The sections were incubated for 3 to 5 minutes with DAB substrate (DakoCytomation, Inc., Carpinteria, CA).
Positive and negative controls were used in every reading and the slides were initially analyzed by one observer that had no previous knowledge about the identification group. In this case, the observer looked for the presence or absence of positivity for each analyzed eye, for both selected markers (GFAP and Calretinin).
When positive for the marker calretinin, two quantitative analysis were performed. In the first analysis, all positive ganglion cells for calretinin in the retina were counted in five consecutive fields, using a 40x objective lens coupled to a 5-head Olympus ® BX50 microscope BX50 (Olympus, Tokyo, Japan). In the second quantitative analysis, the positive areas were marked using the color morphometry method, which consisted of an analysis of the area of the anti-calretinin reaction with the retinal tissue. For this purpose, images of 10 consecutive fields were captured by the 40x objective lens coupled to the BX50 Olympus microscope with the Sony camera, Model DXC-107A, using Image Pro Plus software (Media Cybernetics Inc., Silver Spring, USA). This software allowed an observer to select and paint the positive areas. Moreover, it automatically calculated the area of the positive reaction. The data was entered into a spreadsheet and Microsoft Excel (Redmond, WA) was used to obtain the statistical analysis. The sum of the areas or total area variable, calculated in square micrometers, was related to the sum of all positive areas in the 10 analyzed fields.
Statistical Analysis
The Mann-Whitney nonparametric test was selected to compare the groups in relation to the variables of this study. To compare the analyzed areas, within each group, the Wilcoxon nonparametric test was used. Values of p<0.05 indicated statistical significance.
RESULTS
Lab Results
In basal situation, there was no statistical significance between the groups in relation to glucose, total cholesterol, HDL and triglycerides levels. On Day 14, a significant increase in the glucose and total cholesterol levels of G1 was observed, representing a significant difference in relation to CG. On the euthanasia day, an increase in G1 weight, glucose and total cholesterol levels was observed, making this group significantly different form CG. It is important to point out that the cholesterol level in G1 increased substantially from Day 14 to the euthanasia day (Table 1).
Immunohistochemistry with anti-calretinin
Ganglion Cells
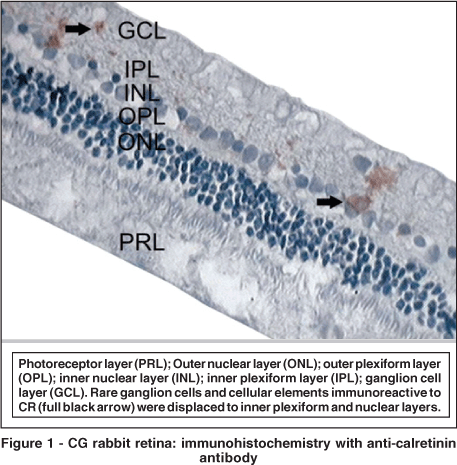
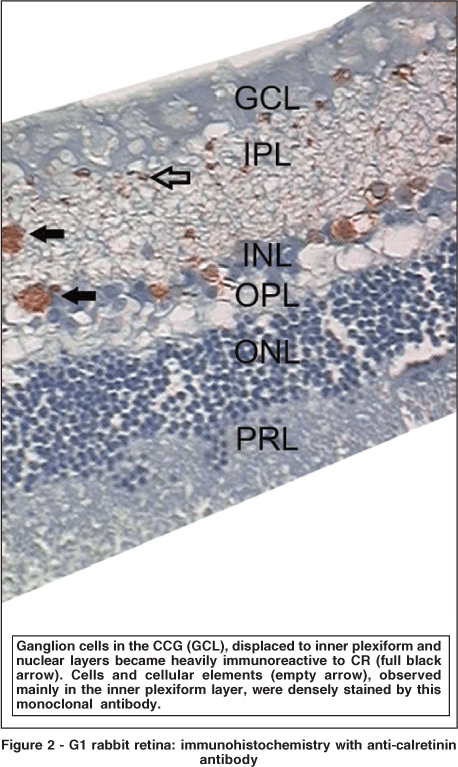
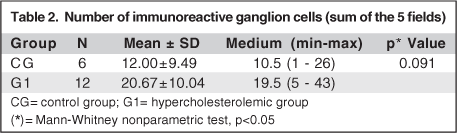
Few ganglion cells in CG reacted to anti-calretinin. They acquired a brownish hue. Some of these cells were displaced to the inner plexiform layer (IPL) and inner nuclear layer (INL) (Figure 1). Conversely, a larger number of immunoreactive ganglion cells was observed in G1 (Figure 2), without statistical relevance (p=0.091) (Table 2). This group also presented displaced ganglion cells in the inner plexiform and nuclear layers.
Color Morphometry - Total anti-calretinin immunoreactive cells (Total area)
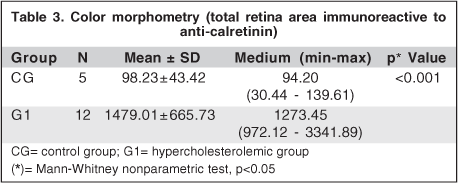
With color morphometry, it was possible to observe that a larger number of cells and cell elements were reactive to anti-calretinin, although this number was significantly lower than in G1 (Table 3). In the hypercholesterolemic group, it was possible to observe, even without color morphometry, a large number of cells immunoreactive to CR (Figure 2). G1 inner plexiform and nuclear layers revealed important immunoreactivity. It is important to point out that it was not possible to perform such an analysis in one of the CG slides. Consequently, only 5 eyes of this group were analyzed using the color morphometry technique.
Immunohistochemistry with GFAP (Glial fibrillary acidic protein)
In both groups, the reaction was negative for all animals.
DISCUSSION
In the present study, the anti-calretinin monoclonal antibody was used to detect early changes of neuronal retinal cells induced by an increase of seric cholesterol. Calretinin is a 31.5 kDa calcium binding protein (CaBPs) belonging to the EF-hand super family. It is considered a predominantly cytosolic protein, although it may also be found in the cell nucleus(14). Some studies attribute to calretinin the role of buffering intracellular Ca(2+), rendering neuroprotection(15-16). Conversely, some experiments claim that CR in neurons does not offer more resistance to calcium overload(17). However, CR can be considered an intracellular calcium ion sensor(12). This monoclonal antibody has already been used to evaluate retinal neuronal cells of normal rabbits(18). In the present study, an analysis of the number of ganglion cells immunoreactive to this monoclonal antibody was first performed as the loss of this cellular type has already been reported in human AMD(19) and associated with Ca(2+) homeostatic imbalance2. G1 rabbits revealed more ganglion cells immunoreactive to this antibody than CG, however, without statistical significance (Table 2), suggesting a trend of cell damage in hypercholesterolemic rabbits. Conversely, the color morphometry analysis performed revealed that the number of immunoreactive cells and cellular elements was significantly higher in G1 than in CG (Table 3). This result indicates that a larger area of cells and cellular elements was stained by anti-calretinin in G1.
The anti-calretinin monoclonal antibody has already been used to study the retinal changes in genetically modified mice models, ApoE-deficient, which were fed a hypercholesterolemic diet for 25 weeks. That study has documented a decrease in or absence of immunoreactivity of these animals retinal cells(2). That result may be attributed to cell apoptosis or death, caused by the chronic increase of seric cholesterol. In this study, opposite results were obtained. G1 has shown reactivity to this immunohistochemical agent significantly higher than CG (Table 3). It is important to point out that all experimental studies performed until this present time, selected to induce hypercholesterolemia for at least 6 months(1-5,9). This time enabled researchers to clearly detect morphologic sclera and choroid changes, whereas, apoptosis, neuronal cell death and reactive gliosis were observed in the retinal tissue. In this study, the detection of the presence of intracellular calcium was crucial, as the time selected to induce hypercholesterolemia was much shorter, 6 weeks, and only early changes, associated with neuronal cell membrane lesion, revealed retinal damage induced by the increase in seric cholesterol.
The histology and morphometry of these animals sclerochoroidal complex revealed a significant increase of G1 histiocytes and collagen fibers when compared to CG, which caused choroid and sclera thickness(20) Such a condition is associated with the decrease in the blood flow into the retina(3,6). The ischemia that took place in the present study in G1 caused Ca (2+) influx into the neuronal retinal cells, but did not cause cell death followed by gliosis. Such findings can explain GFAP negativity in both groups. In CG, absence of immunoreactivity was expected, while in G1 the time of induced hypercholesterolemia was probably insufficient to induce retinal cell death. Consequently the formation of glial tissue did not take place. In other studies, where hypercholesterolemia provided GFAP positivity to Müller glia and made the amacrine cells more immunoreactive, the cholesterol exposure was, at least, 8 months(3-4). In normal conditions, Müller glial cells do not react to this antibody, while amacrine cells are little reactive (21).
A rabbit model was selected over other animals considering the similarities observed between human and rabbits atherosclerosis(22). It is also known that normal serum levels of cholesterol in rabbits vary from 25 to 60 mg% while in humans the levels range from 100 to 200 mg%. Thus, the metabolic system in rabbits can be easily overloaded by a simple daily hypercholesterolemic diet, allowing for more viable experiments and reproducible results(22). This condition has been evidenced in this study. The cholesterol level, in average, increased from 41.50 mg% to approximately 600 mg% in 14 days. At the euthanasia day, cholesterol levels increased to approximately 881 mg%. The rabbit model was also used to evaluate retina, choroid and sclera changes induced by the hypercholesterolemic diet (3-5).
The present study also differs from other studies in the amount of cholesterol administered. G1 animals were fed higher concentration of cholesterol in the first two weeks (1% cholesterol); however, concentrations were the same as the ones presented in the other studies in the last four weeks of the experiment (0.5% cholesterol). This finding has proven to be important scientifically, as it reveals that alterations in the sensory retina of rabbits can be identified in a short period time. Thus, more experiments can be performed and research costs and time can be reduced.
In this study, immunohistochemical analysis was performed with anti-calretinin and GFAP antibodies. Further studies, in similar models, using specific antibodies for iNOS, could demonstrate the retinal abnormality induced by the increase in nitric oxide resultant from the increase accumulation of intracellular calcium.
Finally it is important to point out that early retinal alterations induced by hypercholesterolemia, mainly the ones related to inner retinal layers, have not been studied scientifically until the present time. This study seeks to contribute to the elucidation of the physiopathology of the macular degenerative disease induced by an increase in the seric cholesterol. Consequently, the authors stress the importance of the cardiovascular control of the atherosclerotic disease as an attempt to avoid or reduce the macular damage.
CONCLUSION
The seric cholesterol increase for 6 weeks induced early changes in the sensory retina in rabbits. The anti-calretinin monoclonal antibody had a vital role in the identification of cell changes induced by hypercholesterolemia. Such changes were characterized by calcium deposits in the cytosol.
REFERENCES
1. Miceli MV, Newsome DA, Tate DJ Jr, Sarphie TG. Pathologic changes in the retinal epithelium and Bruch's membrane of fat-fed atherogenic mice. Curr Eye Res. 2000;20(1):8-16.
2. Ong JM, Zorapapel NC, Rich KA, Wagstaff RE, Lambert RW, Rosenberg SE, et al. Effects of cholesterol and apolipoprotein E on retinal abnormalities in ApoE-Deficient mice. Invest Ophthalmol Vis Sci. 2001;42(8):1891-900.
3. Triviño A, Ramirez AL, Salazar JJ, de Hoz R, Rojas B, Padilla E, et al. A cholesterol-enriched diet induces ultrastructural changes in retinal and macroglial rabbit cells. Exp Eye Res. 2006;83(2):357-66.
4. Ramirez AI, Salazar JJ, De Hoz R, Rojas B, Ruiz E, Tejerina T, Ramirez JM, et al. Macroglial and retinal changes in hypercholesterolemic rabbits after normalization od cholesterol levels. Exp Eye Res. 2006;83(6):1423-38.
5. Salazar JJ, Ramirez AI, De Hoz R, Rojas B, Ruiz E, Tejerina T, et al. Alterations in the choroid in hypercholesterolemic rabbits: reversibility after normalization of cholesterol levels. Exp Eye Res. 2007;84(3):412-22.
6. Friedman E. A hemodynamic model of the pathogenesis of age-related macular degeneration. Am J Ophthalmol. 1997;124(5):677-82.
7. Sucher NJ, Lipton SA, Dreyer EB. Molecular basis of glutamate toxicity in retinal ganglion cells. Vision Res. 1997;37(24):3483-93.
8. Montoliu C, Llansola M, Monfort P. Corbalan R, Fernandez-Marticorena I, Hernandez-Viadel ML, et al. Role of nitric oxide and cyclic GMP in glutamate-induced neuronal death. Neurotox Res. 2001;3(2):179-88.
9. Yücel I, Akar Y, Yücel G, Çiftçioglu MA, Keles N, Aslan M. Effect of hypercholesterolemia on inducible nitric oxide synthase expression in a rat model of elevated intraocular pressure. Vision Res. 2005;45(9):1107-14.
10. Vreugdenhil M, Bruehl C, Voskuyl RA, Kang JX, Leaf A, Waldman WJ. Polyunsaturated fatty acids modulate sodium and calcium currents in CA1 neurons. Proc Natl Acad Sci USA. 1996;93(22):12559-63.
11. Puskas LG, Bereczki E, Sántha M, Vigh L, Csanádi G, Spener F, et al. Cholesterol and cholesterol plus DHA diet-induced gene expression and fatty acid changes in mouse eye and brain. Biochimie. 2004;86(11):817-24.
12. Biling-Marczak K, Kuznicki J. Calretinin - sensor or buffer- function still unclear. Pol J Pharmacol. 1999;51(2):173-8.
13. Sun YP, Lu NC, Parmley WW, Hollenbeck CB. Effects of Cholesterol diets on vascular function and atherogenesis in rabbits. Proc Soc Exp Biol Med. 2000; 224(3):166-71.
14. Dechesne CJ, Winsky L, Kim HN, Goping G, Vu TD, Wenthold RJ, et al. Identification and ultrastructural localization of a calretinin-like calcium-binding protein (protein 10) in the guinea pig and rat inner ear. Brain Res. 1991;560(1-2): 139-48.
15. Jande SS, Maler L, Lawson DE. Immunohistochemical mapping of vitamin D-dependent calcium-binding protein in brain. Nature. 1981;294(5843):765-7.
16. Vogt Weisenhorn DM, Weruaga-Prieto E, Célio MR. Calretinin-immunoreactivity in organotypic cultures of the rat cerebral cortex: effects of serum deprivation. Exp Brain Res. 1996;108(1):101-12.
17. Mockel V, Fischer G. Vulnerability to excitotoxic stimuli of cultured rat hippocampal neurons containing the calcium-binding proteins calretinin and calbidin D28K. Brain Res. 1994;648(1):109-20.
18. Völgyi B, Pollak E, Buzás P, Gabriel R. Calretinin in neurochemically well-defined cell populations of rabbit retina. Brain Res. 1997;763(1):79-86
19. Ramirez JM, Triviño A, Ramirez AI, Salazar JJ, García-Sánchez J. Structural specializations of human retinal glial cells. Vision Res. 1996;36(14):2029-36.
20. Torres RJ, Maia M, Noronha L, Farah ME, Luchini A, Brik D, et al. [Evaluation of choroid and sclera early alterations in hypercholesterolemic rabbits: histologic and histomorphometric study]. Arq Bras Oftalmol. 2009;72(1):68-74. Portuguese
21. Schnitzer J. Astrocytes in the guinea pig, horse, and monkey retina: their occurrence coincides with the presence of blood vessels. Glia. 1988;1(1):74-89.
22. Cogan DG, Kuwabara T. Ocular changes in the experimental hypercholesterolemia. Arch Ophthalmol. 1959;61:219-25.
 Correspondence adress:
Correspondence adress:
Rogil José de Almeida Torres
Rua Emiliano Perneta, 390 - Conj. 1.411
Curitiba (PR) CEP 80420-080
E-mail: [email protected]
Recebido para publicação em 10.03.2009
Última versão recebida em 07.11.2009
Aprovação em 09.11.2009
Participating Institutions: Hospital Angelina Caron - Campina Grande do Sul - Curitiba (PR) - Brazil; Ophthalmology Department at the Universidade Federal de São Paulo - São Paulo (SP) - Brazil; Pontifícia Universidade Católica do Paraná - Curitiba (PR) - Brazil.
Este trabalho faz parte da tese de Doutorado em Ciências da Visão - UNIFESP - São Paulo (SP) - Brazil.