

Gildasio Castello de Almeida Junior1; Fabio Batista Frederico2; Karina Paula Watanabe2; Tatiana Vanucci Garcia2; Ângela Yuri Iquejiri2; Patrícia Maluf Cury3; Sebastião Roberto Taboga4; Reinaldo Azoubel5
DOI: 10.1590/S0004-27492008000400019
ABSTRACT
PURPOSE: To evaluate the effectiveness of mitomycin C (MMC) in preventing recurrence of pterygium following conjunctival autograft transplantation (CAT). Ki-67 antigen to evaluate epithelial cell proliferation and fibroblast nuclear kariometry were used to assist treatment evaluation. METHODS: Twenty-nine patients with recurrent pterygium were divided into three groups: Group (G) 1 - CAT and placebo eyedrops (PED); G2 - CAT, 0.015% MMC subconjunctivally, and PED; G3 - CAT and 0.02% MMC eyedrops. Immunohistochemistry for the Ki-67 antigen and fibroblast nuclei kariometry were performed on the excised tissue, divided into nasal and temporal sides. Kariometry was evaluated in terms of volume (Vl) and area (Ar) using at least 50 cells/patient. RESULTS: The percentage of positive epithelial cells for the Ki-67 antigen on the nasal and temporal side after treatment of the three groups were: nasal (5.39% G1, 4.49% G2, and 3.88% G3); temporal (3.30% G1, 4.46% G2, 4.14% G3), did not show significant differences. Fibroblast nucleus kariometry was: nasal Vl (792.1 µ3 G1, 605.1 µ3 G2, and 549.9 µ3 G3) and Ar (100.58 µ2 G1, 83.13 µ2 G2, and 78.41 µ2 G3). The three groups showed significant differences: p=0.039 and p=0.035, for respectively Vl and Ar, on the nasal side. After a six month of treatment, the three groups presented the following recurrence rates: G1, 22.22%, G2, 18.18% and G3, 33.33%, respectively. CONCLUSION: MMC did not reduce the number of positive epithelial cells for the Ki-67 antigen in recurrent pterygium, but decreased fibroblast nucleus volume and area on the nasal side of the pterygia. The number of positive epithelial cells for the Ki-67 antigen seemed not to be related to pterygium recurrence observed over a six-month post-surgery period. The role of epithelial cell proliferation in pterygium recurrence should be evaluated by further studies.
Keywords: Ki-67 antigen; Pterygium; Recurrence; Mitomycin; Epithelial cells; Fibroblasts; Karyometry
RESUMO
OBJETIVO: Avaliar a eficácia da mitomicina C (MMC) na prevenção da recorrência quando previamente utilizada no transplante autólogo de conjuntiva (TAC). A avaliação da proliferação celular epitelial pelo antígeno Ki-67 e a cariometria do núcleo dos fibroblastos foram usados como auxiliares na avaliação do tratamento. MÉTODOS: Vinte e nove pacientes com pterígio recidivado foram divididos em três grupos: Grupo (G) 1-TAC e colírio placebo (PLA); G2-TAC, MMC 0,015% subconjuntival e PLA; G3-TAC e colírio de MMC 0,02%. A imuno-histoquímica foi realizada no tecido excisado para o antígeno Ki-67, como a cariometria dos núcleos dos fibroblastos (divididos em lado nasal e temporal). A cariometria dos núcleos dos fibroblastos foi avaliada de acordo com os seguintes parâmetros: volume (Vl) e área (Ar) em pelos menos 50 células por paciente. RESULTADOS: A porcentagem das células epiteliais positivas para o antígeno Ki-67 no lado nasal e temporal após o tratamento dos três grupos estudados foi: nasal (3,30% G1, 4,49% G2 e 3,38% G3) e temporal (3,30% G1, 4,46% G2 e 4,14% G3) não mostrando diferença significativa. A cariometria do núcleo dos fibroblastos foi: Vl nasal (792,1 µ3 G1, 605,1 µ3 G2, e 549,9 µ3 G3) e a Ar (100,58 µ2 G1, 83,13 µ2 G2, e 78,41 µ2 G3). Os três grupos mostraram uma diferença significativa p=0,039 e p=0,035, respectivamente do Vl e da Ar no lado nasal. Após seis meses de tratamento, os três grupos apresentaram a seguinte taxa de recidiva: 22,22% G1, 18,18%, G2 e 33,33% G3 respectivamente. CONCLUSÃO: O uso da MMC não interferiu nas células epiteliais positivas para o antígeno Ki-67 no pterígio recidivado, mas acarretou diminuição do volume e área dos núcleos dos fibroblastos no lado nasal do pterígio. As células epiteliais positivas para o antígeno Ki-67 parecem não ter relação com a recidiva do pterígio após seis meses da cirurgia. Outros estudos devem ser realizados para avaliar o papel da proliferação das células epiteliais na recorrência do pterígio.
Descritores: Antígeno Ki-67; Pterígio; Recidiva; Mitomicina; Células epiteliais; Fibroblastos; Cariometria
ORIGINAL ARTICLE
Evaluation of epithelial cell proliferating activity and fibroblast nuclear kariometry in recurrent pterygium treated with mitomycin C
Avaliação da atividade proliferativa das células epiteliais e da cariometria do núcleo dos fibroblastos em pterígios recidivados previamente tratados com a mitomicina C
Gildasio Castello de Almeida JuniorI; Fabio Batista FredericoII; Karina Paula WatanabeII; Tatiana Vanucci GarciaII; Ângela Yuri IquejiriII; Patrícia Maluf CuryIII; Sebastião Roberto TabogaIV; Reinaldo AzoubelV
IDoutor, Médico Assistente do Setor de Córnea da Faculdade de Medicina de São José do Rio Preto - FAMERP - São José do Rio Preto (SP) - Brasil
IIResidente em Oftalmologia da FAMERP - São José do Rio Preto (SP) - Brasil
IIIDoutora, Professora do Departamento de Patologia da FAMERP - São José do Rio Preto (SP) - Brasil
IVLivre Docente, Professor Adjunto do Departamento de Biologia da Universidade Estadual Paulista "Júlio de Mesquita Filho" - UNESP - São José do Rio Preto (SP) - Brasil
VLivre Docente, Professor e Coordenador do Programa de Pós-graduação da FAMERP - São José do Rio Preto (SP) - Brasil
ABSTRACT
PURPOSE: To evaluate the effectiveness of mitomycin C (MMC) in preventing recurrence of pterygium following conjunctival autograft transplantation (CAT). Ki-67 antigen to evaluate epithelial cell proliferation and fibroblast nuclear kariometry were used to assist treatment evaluation.
METHODS: Twenty-nine patients with recurrent pterygium were divided into three groups: Group (G) 1 - CAT and placebo eyedrops (PED); G2 - CAT, 0.015% MMC subconjunctivally, and PED; G3 - CAT and 0.02% MMC eyedrops. Immunohistochemistry for the Ki-67 antigen and fibroblast nuclei kariometry were performed on the excised tissue, divided into nasal and temporal sides. Kariometry was evaluated in terms of volume (Vl) and area (Ar) using at least 50 cells/patient.
RESULTS: The percentage of positive epithelial cells for the Ki-67 antigen on the nasal and temporal side after treatment of the three groups were: nasal (5.39% G1, 4.49% G2, and 3.88% G3); temporal (3.30% G1, 4.46% G2, 4.14% G3), did not show significant differences. Fibroblast nucleus kariometry was: nasal Vl (792.1 µ3 G1, 605.1 µ3 G2, and 549.9 µ3 G3) and Ar (100.58 µ2 G1, 83.13 µ2 G2, and 78.41 µ2 G3). The three groups showed significant differences: p=0.039 and p=0.035, for respectively Vl and Ar, on the nasal side. After a six month of treatment, the three groups presented the following recurrence rates: G1, 22.22%, G2, 18.18% and G3, 33.33%, respectively.
CONCLUSION: MMC did not reduce the number of positive epithelial cells for the Ki-67 antigen in recurrent pterygium, but decreased fibroblast nucleus volume and area on the nasal side of the pterygia. The number of positive epithelial cells for the Ki-67 antigen seemed not to be related to pterygium recurrence observed over a six-month post-surgery period. The role of epithelial cell proliferation in pterygium recurrence should be evaluated by further studies.
Keywords: Ki-67 antigen; Pterygium/drug therapy; Recurrence; Mitomycin/therapeutic use; Epithelial cells; Fibroblasts; Karyometry
RESUMO
OBJETIVO: Avaliar a eficácia da mitomicina C (MMC) na prevenção da recorrência quando previamente utilizada no transplante autólogo de conjuntiva (TAC). A avaliação da proliferação celular epitelial pelo antígeno Ki-67 e a cariometria do núcleo dos fibroblastos foram usados como auxiliares na avaliação do tratamento.
MÉTODOS: Vinte e nove pacientes com pterígio recidivado foram divididos em três grupos: Grupo (G) 1-TAC e colírio placebo (PLA); G2-TAC, MMC 0,015% subconjuntival e PLA; G3-TAC e colírio de MMC 0,02%. A imuno-histoquímica foi realizada no tecido excisado para o antígeno Ki-67, como a cariometria dos núcleos dos fibroblastos (divididos em lado nasal e temporal). A cariometria dos núcleos dos fibroblastos foi avaliada de acordo com os seguintes parâmetros: volume (Vl) e área (Ar) em pelos menos 50 células por paciente.
RESULTADOS: A porcentagem das células epiteliais positivas para o antígeno Ki-67 no lado nasal e temporal após o tratamento dos três grupos estudados foi: nasal (3,30% G1, 4,49% G2 e 3,38% G3) e temporal (3,30% G1, 4,46% G2 e 4,14% G3) não mostrando diferença significativa. A cariometria do núcleo dos fibroblastos foi: Vl nasal (792,1 µ3 G1, 605,1 µ3 G2, e 549,9 µ3 G3) e a Ar (100,58 µ2 G1, 83,13 µ2 G2, e 78,41 µ2 G3). Os três grupos mostraram uma diferença significativa p=0,039 e p=0,035, respectivamente do Vl e da Ar no lado nasal. Após seis meses de tratamento, os três grupos apresentaram a seguinte taxa de recidiva: 22,22% G1, 18,18%, G2 e 33,33% G3 respectivamente.
CONCLUSÃO: O uso da MMC não interferiu nas células epiteliais positivas para o antígeno Ki-67 no pterígio recidivado, mas acarretou diminuição do volume e área dos núcleos dos fibroblastos no lado nasal do pterígio. As células epiteliais positivas para o antígeno Ki-67 parecem não ter relação com a recidiva do pterígio após seis meses da cirurgia. Outros estudos devem ser realizados para avaliar o papel da proliferação das células epiteliais na recorrência do pterígio.
Descritores: Antígeno Ki-67; Pterígio/quimioterapia; Recidiva; Mitomicina/uso terapêutico; Células epiteliais; Fibroblastos; Cariometria
INTRODUCTION
Pterygium is a highly vascularized, proliferating triangular fleshy mass of thickened conjunctiva extending from the inner corner of the eye to the nasal border of the cornea(1). Its substantia propria shows an elastolic degeneration of collagen, exhibiting elastodysplasia and elastodystrophy(2). Recent studies have shown that this pathology results from a deregulation of the healing process, in which molecular events leading to apoptosis are modified(3-5). Pterygium may constitute a degenerative disease(2,6), since many studies have shown the presence of matrix metalloproteinases (MMPs)(7-9) in it.
Pterygium incidence is higher among persons aged between 20 to 40 years(9-10), in those undergoing high-level exposure to ultraviolet (UV) light(11-12), and UV B was shown to be a risk factor for the development of the anomaly(13).
Recurrent or secondary pterygium is often present as a growing fibrovascular tissue, more exuberant than the primary one(10). Histological findings differ from those of primary pterygium by not showing typical changes of degenerating connective tissue(10). The strong immunoreactivity and release of the basic fibroblast growth factor (b-FGF) in cultured fibroblasts of recurrent pterygia, suggest that these cells play an important role in pterygium recurrence(14).
The classical treatment for pterygium consists of its resection followed by the use of conjunctival, amniotic membrane or other type of graft to cover the exposed scleral bed. In the conjunctival autograft transplantation (CAT) technique, described by Kenyon et al. in 1985(15), a graft is initially released from the superior bulbar conjunctiva and subsequently used to cover the exposed sclera following resection.
The topical use of mitomycin C (MMC) to prevent pterygium recurrence was first described in Japan in the early 1960s(16). The main DNA target for the covalent connection of MMC is in position N2 of guanine(17). This action is responsible for its antibiotic and antineoplastic activities, because it usually represses DNA replication. Donnenfeld et al.(18) evaluated the safety and effectiveness of therapeutic MMC using a dose of 0.1 ml of 0.15 mg/ml MMC, prior to surgery, obtaining a pterygium recurrence rate of 6%, although intraoperative MMC and postoperative MMC are two of the methods of adjunctive therapy that have been most commonly reported recently(19).
The present study was aimed at a comparison of the effectiveness of preventing subsequent recurrence of pterygium, by topical MMC eyedrop or subconjunctival administration previous to CAT surgery. Epithelial cell proliferation estimated by the detection of the Ki-67 antigen, as well as evaluation of conjunctival fibroblast kariometry were also performed.
METHODS
Subjects
Twenty-nine patients showing recurrent nasal pterygium, referred to the Ophthalmology Service of FAMERP (Faculty of Medicine of São José do Rio Preto - São Paulo State), were enrolled in the study. Criteria for eligibility were: 1 - pterygium growth of more than 3 mm over the cornea, horizontally from the anatomical limbus with or without ocular symptoms (irritation, photophobia, burning pain); 2 - absence of connective tissue disease capable to influence wound healing; 3 - no known allergy to MMC; 4 - no known trauma to the affected eye; 5 - recurrence of nasal pterygium. Patients were randomly divided into three groups: Group 1 - Nine patients submitted to CAT, using placebo eyedrops (PED) for 14 days prior to surgery. Group 2 - Eleven patients submitted to CAT and subconjunctival injection of 0.1 ml of 0.015% MMC(18) and PED in the pterygium head 30 and 14 days prior to surgery, respectively. Group 3 - Nine patients submitted to CAT, using 0.02% MMC eyedrops for 14 days before surgery. Placebo and MMC eyedrops were prepared by the Ophthalmos® Laboratory (São Paulo, Brazil).
The pterygia were graded according to the system used by Tan et al.(20); grade 1 (atrophic), episcleral vessels under the body of the pterygium are not obscured and clearly distinguished; grade 3 (fleshy), episcleral vessels totally obscured; and grade 2 (intermediate), all other pterygia not falling into these 2 grades. Patients of the study were classified as grade 3. Pterygium size and vascularization were not taken into account; however, the number of their previous recurrences was considered. Patients were followed for six months after surgery.
Study conditions complied with those of the Helsinki declaration(21); patients were informed about them and signed a consent form approved by the FAMERP Ethics Committee on Research Protocols(22) # 003186/04 and Current Controlled Trial # ISRCTN82504082.
Exclusion Criteria:
• Patients showing keratoconjunctivitis sicca, acne rosacea, neurothrophic keratopathy, and severe dysfunction of the meibomian glands.
• Patients using any immunosuppressive drug administered systemically topically.
• Individuals under 18 years of age.
• Populations exhibiting vulnerability due to physical or mental infirmity, language difficulties or membership of minority groups like for example, ethical origin or sexuality, having associated glaucoma, or using ocular hypotensives.
Surgical technique:
Surgery with CAT was carried out as described by Kenyon et al.(15). All patients underwent peribulbar anesthesia performed by the same surgeon (GCAJ) assisted by second-year medical residents. CAT was carried out by dissection with a no. 15 slide blade. The cornea was isolated in a way permitting fibrovascular tissue to be excised up to the medial rectum insertion. Thermal cauterization was not performed in any patient; rather, the bare scleral area was abundantly irrigated with balanced saline solution until bleeding stopped. The size of the bare sclera area was measured with a Castrovejo compass; one millimeter larger grafts were released from the bulbar super temporal conjunctiva, and carefully placed on the sclera to preserve the limbus-limbus orientation. Continuous suturing was performed with mononylon 10-0 and the suture anchored on the episclera at the corners of the rectangle formed by the graft. A mononylon 10-0 stitch was passed in the graft center on the same side of the limbus, to increase adherence, and prevent the free graft from moving forward on the cornea.
Patients were examined postoperatively on the first, seventh, and thirtieth day, and after six months. Postsurgical drug treatment included the use of eyedrops with 0.3 mg/ml tobramycin and 0.1 mg/ml dexamethasone, and an 0.1 mg/ml dexamethasone ointment.
Corneal recurrence was defined as any growth of conjunctiva into the clear cornea, evaluated from the anatomical limbus. Conjunctival recurrence, invasion of conjunctival fibrovascular tissue inside the graft was not evaluated.
Excised tissue of the pterygium was fixed on nitrocellulose paper with indication of the orientations of the nasal and temporal sides, placed in a phosphate buffered solution of 10% formol and sent to the FAMERP Laboratory of Histopathology, embedded in paraffin. Four sections were taken, each five microns thick, separated by 45-microns, and fixed on a slide blade with silane to divide the nasal and temporal sides. The blades were sent to the Laboratory of Pathology at the Base Hospital for immunohistochemical analysis, and to the FAMERP Laboratory of Histology for evaluation of fibroblast kariometry.
Evaluation of fibroblast kariometry
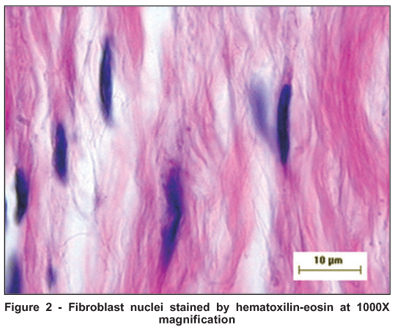
The material was split into semi-serial six-micron sections, and stained with hematoxilin-eosin. A Hund H500 Wetzlar (Helmut Hund Gmbh, Germany) optical microscope with immersion lenses (1000X), was used to estimate the nuclear parameters of the fibroblast cells, a technique first validated by Chalkley in 1943(23).
Projections of nuclei were made on white paper at a final magnification of 1240X. Fifty nuclear images from each patient of each group (nasal and temporal side), were then outlined with a black #2 pencil, carefully recording only the elliptical images. A millimeter-scale ruler was used to measure their larger (D) and smaller axes (d) and the diameters were recorded. The following nuclear parameters were then used to determine area and volume:
Volume: V= π/6. M3
Area: A= π/6. M2
Immunohistochemical analysis
The slide blades were heated in an oven at 60ºC, for 30 minutes, deparaffinized in xylol for 30 minutes at room temperature, and placed in vats containing 3% H2O2 in methanol, to block endogenous peroxidase activity. Samples were then incubated with Ki-67 antigen (Novocastra, Newcastle, United Kingdom) diluted 1:400 in a 100-µl solution of 1% albumin and 1% sodium azide in PBS (16.36 g NaCl + 2.88 g dibasic sodium phosphate + 0.434 g monobasic sodium phosphate), dissolved in two liters of distilled water, adjusting the pH with NaOH and HCl to 7.4, for 1 hour at 37ºC in a humid chamber. After a threefold washing with PBS for 5 minutes, samples were first incubated with biotinylated antibody LSAB+ System-HRP (DakoCytomation, Glostrup, Denmark) and then with 100 µl biotin-streptavidin-peroxidase compound (Streptabe), in a humid chamber for 30 minutes at 37ºC, immersed in this solution for three to five minutes at 37ºC, in the dark, and washed with distilled water. Excess was kept in running water according to Shi et al.(24). Negative and positive controls were run in the immunohistochemical analyses.
Evaluation of the proliferating activity of epithelial cells
This activity was followed on the blades, at microscopic 400X magnification. At least 500 epithelial cells were counted; cells showing a brown nucleus were considered positive for the Ki-67 antigen. The total number of positive cells was calculated and divided by the total counted number of positive and negative cells, thus permitting the determination of percent values for each patient. The evaluation of epithelial cells was performed by a single examiner who had no knowledge of the examined group identity.
Data analysis
Data were analyzed using Minitab 13 software (Statistical Software for Windows, State College, Pennsylvania, USA). Kruskal-Wallis non-parametric test was used to evaluate the percentage of proliferating epithelial cells that were positive for the Ki-67 antigen. Wilcoxon´s test was used to compare mitotic activity of nasal and temporal sides of each group of cells, using Ki-67 antigen as indicator. The Mann-Whitney test was used to evaluate (independently of group); the percentage of proliferating cells was determined using the Ki-67 antigen, among patients showing or not pterygium recurrence. Kruskal-Wallis's non-parametric test was used to compare the areas and volumes of the fibroblast nuclei of the three groups. A 0.05 = or < significance level was considered valid for the results of all effected comparison.
RESULTS
Table 1 shows average age and number of previous recurrences prior to surgery treatment, of the three studied groups.

Figure 1 shows the increase at 400 X magnification, of the nuclei of epithelial cells stained brown by the Ki-67 antigen, of the three studied groups. No significant differences between the proliferative activity of the epithelial cells of the three groups, evaluated by medians of the percentage of positive cells for the Ki-67 antigen, was observed either on the nasal (p=0.923) (Figure 1), or the temporal side (p=0.447) (Table 2). However, a greater variation on the nasal and temporal sides of the strength of the Ki-67 antigen stain of the median of Group 1 relative to those of the other groups was noted (Table 2).
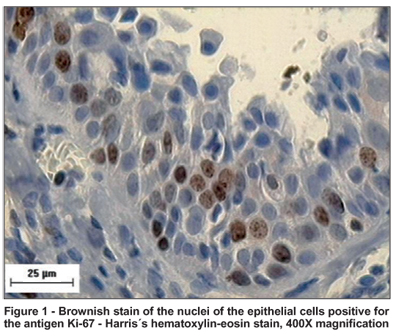
Table 2 shows that the Wilcoxon test showed no significant differences when the percentages of positive epithelial cells assessed by the Ki-67 antigen on the nasal and temporal sides of each group were compared.
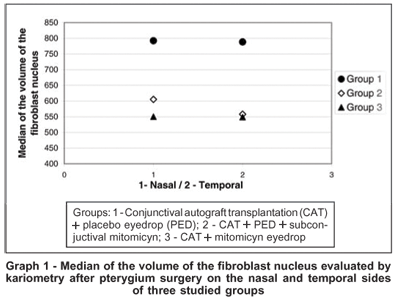
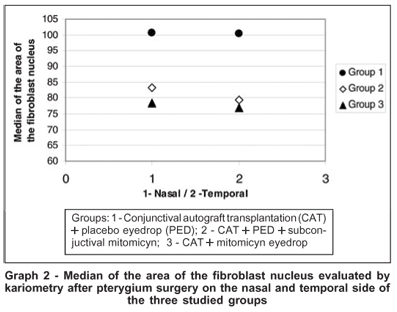
The median of area and volume of the fibroblast nucleus evaluated by kariometry at 1000X optical microscope magnification (Figure 2), counting at least 50 cells per field, showed a decrease in groups 2 and 3 when compared to the controls, occurring on both the nasal and temporal sides of the excised tissue; however, the nasal side showed significant differences when the three groups were compared in relation to volume (H=6.48, p=0.039) and area (H=6.73, p=0.035), respectively (Table 3). Furthermore, Group 3 which used topical MMC for 14 days prior to surgery, still showed lower values for average fibroblast nuclear area and volume when compared with Groups 1 and 2 (Graphs 1 and 2); this result was more marked on the nasal side.
There was no correlation between the positive epithelial cells for the Ki-67 antigen of patients that had shown recurrence and those that had not, at the nasal side (W=360.0, p = 0.13), and at the temporal side (W=309.0, p=0.29).
After six months of treatment, Group 1 presented a recurrence rate of 22.22%, whereas Groups 2 and 3 presented recurrence rates of 18.18% and 33.33%, respectively. No patient presented any postoperative complication when using MMC.
DISCUSSION
The distribution of the average age and the number of previous recurrences of the treatment were similar in the three studied groups (Table 1). The number of previous recurrences prior to treatment was higher in Group 2. The average ages of the three groups at the beginning of the study were higher than those described in the literature; however, this may have been due to this population group being composed of patients with recurrent pterygia(9-10).
Figure 1 shows that a higher level of positivity for Ki-67 antigen was shown by epithelial cells situated close to the basement membrane. This indicates that the basal cells of the epithelium exhibit high levels of mitosis. Despite the fact that the subepithelial layer was not evaluated in this study, it is important to point out that proliferative cell activity in the conjunctival subepithelial layer of the three groups had occurred.
The use of MMC, either subconjuntively or topically, did not alter the percentage of positive epithelial cells to Ki-67 antigen in the control group, either on the nasal (p=0.923) or the temporal sides (p=0.447). However, with the use of MMC, the proliferative activity in the epithelial cells may have been lower. Thus, the vascular endothelium and in fibroblasts previously treated with subconjunctival MMC showed a histopathological alkylating chemotherapeutic effect(18). There is a higher proliferative activity using the Ki-67 antigen in squamous cell carcinomas and in intraepithelial neoplasia of the conjunctiva compared to the pterygium(25).
One study found the percentage of positive cells to Ki-67 antigen in the pterygium tissue to be 9.0 ± 2.2%; in our study it was close to 4.0% in the three groups, even when patients who did not use MMC prior to surgery were included(25).
No significant difference was observed when the nasal side was compared with the temporal side of the pterygia (Table 2); however, there is substantial mitotic activity in the limbus area of the ocular surface(26), slightly more evident in the nasal side of group 1 (Table 2). There is strong indication that pterygium arises in this area(27).
After a six-month follow-up, no significant difference was observed in relation to the percentage of positive epithelial cells for the Ki-67 antigen of patients who did not present recurrence were compared with those presenting recurrence, independently of the group in wich each patient was classified in the begining of the study, either on the nasal (p=0.11) or the temporal side (p=0.61). Recently, two studies reported high expression of p53 in the epithelium of the pterygium, suggesting a mutation of the p53 gene(4-5). However, the mutation of p53 is not important for the formation or recurrence of pterygia(28). No other published studies evaluated the positive correlation of the epithelial cells of the pterygium for the Ki-67 antigen with the pterygium's recurrence rate.
The recurrence rate for surgical treatment of the pterygium with CAT is about 2% to 9%(15, 29-30); however, there are reports stating that this rate may range between 2% and 39%(15,29-31). In our Group 3, a rate of 33.33% was observed, a value that was higher than in the other groups. Group 2 presented a lower recurrence rate, 18.88%; this group also showed a higher number of patients with recurrences prior to surgery. Despite the small sample size, and a follow-up period of less than one year, the lower recurrence rate in Group 2, possibly corroborates the results of a recurrence rate of an other study that used subconjunctival MMC(18). Furthermore, another study(32) that evaluated 36 patients with recurrent pterygia after a 6-month follow-up, showed a 41.1% recurrence rate, with 20% of these patients showing a recurrence between 3 and 6 months postoperatively, although no adjuvant therapy was used, the outcome was similar to that of the present study. Yet, another study found a recurrence rate of 4.16% in patients with recurrent pterygium, a much lower percentage than that found in the present study(33). Despite the short, six-month follow-up period of the patients in the present study and the use of MMC, none presented the postoperative complications reported by some authors. For instance, mild complications due to excessively low conjunctival vascularization after topical use of MMC was described(34), and ten cases were described that had serious complications, including edema and perforation of the cornea and scleral calcification, associated with MMC use after pterygium surgery(35). Nevertheless, it is important to emphasize that MMC is considered a long-term "radiomimetic agent", causing permanent effects on tissues(36); its topical use can alter the tear film stability, goblet cell loss, and increase in squamous metaplasia(37). MMC related changes may persist in the ocular surface epithelium for at least 8 months following therapy(38).
A characteristic feature of pterygia is the loss of BL (Bowman's layer), and the mechanism responsible for this destruction in pterygia is currently unknown(39). Overexpression of MMPs and tissue inhibitors of MMPs (TIMPs) by pterygial epithelial cells in regions without BL, suggests that these proteins may contribute to matrix remodeling and BL destruction. Taken together, these data suggest that the epithelial cell is the most likely candidate involved in the dissolution of BL(39). It is therefore tempting to speculate that in situ production of collagenase-1 and gelatinase A by epithelial cells may be partially responsible for the extensive collagen degradation in pterygia(7). The use of MMC in the present study had a low effect on epithelial cell activity over time, since no significant differences of the recurrence rate in the groups treated with MMC could be explained by the absence of differences in proliferative epithelial cell activity. The relationship between the role of mitotic activity of the epithelial cells by Ki-67 antigen and MMPs and TIMPs is not known; however, it is probable that at high epithelial cell activity, conjunctival tissue should have higher amounts of MMPs.
A decrease in the area and volume of fibroblast nuclei mainly in the nasal area of the pterygium was observed in groups treated with MMC (Graphs 1 and 2). As shown in Table 3, only the nasal side showed a significant decrease in nuclear volume and area. We were unable to find reports on the kariometry of the fibroblast nucleus in pterygial cases treated with MMC. The importance of evaluating these fibroblast variables is that these cells can activate myofibroblasts through fibrogenic stimulation, by transforming growth factor b, connective tissue growth factor and platelet-derived growth factor(40), present in pterygia(41). Moreover, normal tissue myofibroblasts may become activated and migrate to the pterygial tissue(42): thus, the presence of myofibroblasts in both the periorbital fibrous tissue and that posterior to Tenon's capsule, close to the nasal conjunctive, could lead to pterygium recurrence(42). Fibroblasts may have an important role in epithelium differentiation, since collagen fibrillae in the interstitial space indicate fibroblastic activity, and are never observed in epithelium free from pterygia(43).
Despite the fact that the volumes and nuclear areas of fibroblasts were smaller on the nasal side of the treated groups, this decrease was clinically unrelated to the lower rate of recurrence in these groups; it is however important to note the timing of the follow-up, as well as the number of samples used in our study. The tissue phenotype depends on an interaction between the extracellular matrix and the structural organization of the nucleus(44). In addition, it is not known whether the decrease in area and volume of the fibroblast nuclei interferes with pterygium recurrence. The expression of MMP-1 and MMP-3 by fibroblasts of the pterygial body in culture, following stimulation by proinflammatory mediators like interleukin 1 (IL-1) and tumor necrosis factor a (TNF-α), increased in certain fibroblast types, demonstrating the inflammatory role of pterygium pathogenesis(8). After the fibroblast nucleus was treated with MMC, either topically or subconjunctival, it showed a smaller volume and area than that of the control group of this study. Are these nuclei less influenced by proinflammatory mediators to express MMP? One source of IL-1 and TNF-a is the stromal fibroblast(45), which also produces inhibitory factors of MMPs (46) like for example, the tissue inhibitor of metalloproteinases (TIMPs). Could this change in the fibroblast nucleus have a prolonged effect on expressing MMP?
CONCLUSIONS
MMC used by the subconjunctival or topical routes did not alter the percentage of conjunctival positive epithelial cells for the Ki-67 antigen in recurrent pterygia. The percentage of positive epithelial cells for the Ki-67 antigen may not bear a relationship to pterygium recurrence shown six months after surgery. Kariometry showed a significant decrease in the volume and area of the fibroblast nuclei on the pterygium nasal side in groups treated with MMC. However, the decrease in volume and area of the fibroblast nuclei had no clinical effect on the reduction of pterygium recurrence rate after a six-month follow-up period. Further studies should be performed to evaluate the role of epithelial cell proliferation and fibroblast nucleus kariometry on pterygium recurrence.
REFERENCES
1. Dushku N, Reid TW. Immunohistochemical evidence that human pterygia originate from an invasion of vimentin-expressing altered limbal epithelial basal cells. Curr Eye Res. 1994;13(7):473-81.
2. Austin P, Jakobiec FA, Iwamoto T. Elastodysplasia and elastodystrophy as the pathologic bases of ocular pterygia and pinguecula. Ophthalmolology. 1983; 90(1):96-109.
3. Tan DT, Tang WY, Liu YP, Goh HS, Smith DR. Apoptosis and apoptosis related gene expression in normal conjunctiva and pterygium. Br J Ophthalmol. 2000;84(2):212-6.
4. Dushku N, Reid TW. P53 expression in altered limbal basal cells of pingueculae, pterygia, and limbal tumors. Curr Eye Res. 1997;16(12):1179-92.
5. Tan DT, Lim AS, Goh HS, Smith DR. Abnormal expression of the p53 tumor suppressor gene in the conjunctiva of patients with pterygium. Am J Ophthalmol. 1997;123(3):404-5.
6. Wang IJ, Hu FR, Chen PJ, Lin CT. Mechanism of abnormal elastin gene expression in the pinguecular part of pterygia. Am J Pathol. 2000;157(4): 1269-76.
7. Di Girolamo N, McCluskey P, Lloyd A, Coroneo MT, Wakefield D. Expression of MMPs and TIMPs in human pterygia and cultured pterygium epithelial cells. Invest Ophthalmol Vis Sci. 2000;41(3):671-9.
8. Solomon A, Li DQ, Lee SB, Tseng SC. Regulation of collagenase, stromelysin, and urokinase-type plasminogen activator in primary pterygium body fibroblasts by inflammatory cytokines. Invest Ophthalmol Vis Sci. 2000; 41(8):2154-63.
9. Anduze AL, Merritt JC. Pterygium: clinical classification and management in Virgin Islands. Ann Ophthalmol. 1985;17(1):92-5.
10. Hill JC, Maske R. Pathogenesis of pterygium. Eye. 1989;3(Pt 2):218-26. Review.
11. Cameron ME. Pterygium throughout the world. In: Thomas C, editors. Illinois: Springfield; 1965. p.141-71.
12. Moran DJ, Hollows FC. Pterygium and ultraviolet radiation: a positive correlation. Br J Ophthalmol. 1984;68(5):343-6.
13. Coroneo MT. Pterygium as an early indicator of ultraviolet insolation: a hypothesis. Br J Ophthalmol. 1993;77(11):734-9.
14. Kria L, Ohira A, Amemiya T. Growth factors in cultured pterygium fibroblasts: immunohistochemical and ELISA analysis. Graefes Arch Clin Exp Ophthalmol. 1998;236(9):702-8.
15. Kenyon KR, Wagoner MD, Hettinger ME. Conjunctival autograft transplantation for advanced and recurrent pterygium. Ophthalmology. 1985;92(11): 1461-70.
16. Kunitomo N, Mori S. Studies on the pterygium. Part IV. A treatment of the pterygium by mitomycin-C instillation. Acta Soc Ophthalmol Jpn. 1963;67:601-7.
17. Dorr RT. New findings in the pharmacokinetic, metabolic, and drug-resistance aspects of mitomycin C. Semin Oncol. 1988;15(3 Suppl 4):32-41.
18. Donnenfeld ED, Perry HD, Fromer S, Doshi S, Solomon R, Biser S. Subconjunctival mitomycin C as adjunctive therapy before pterygium excision. Ophthalmology. 2003;110(5):1012-6. Comment in: Ophthalmology. 2003; 110(11):2257-8; author reply 2258.
19. Hirst LW. The treatment of pterygium. Surv Ophthalmol. 2003;48(2):145-80. Comment in: Surv Ophthalmol. 2004;49(1):129-30; author reply 130. Surv Ophthalmol. 2004;49(3):376; author reply 376. Surv Ophthalmol. 2004;49(5): 541-2; author reply 542-3.
20. Tan DT, Chee SP, Dear KB, Lim AS. Effect of pterygium morphology on pterygium recurrence in a controlled trial comparing conjunctival autografting with bare sclera excision. Arch Ophthalmol. 1997;115(10):1235-40. Erratum in: Arch Ophthalmol. 1998;116(4):552.
21. The World Medical Association. World Medical Association Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects [Internet]. [cited 2007 Oct 26]. Available from: http://www.wma.net/e/policy/pdf/17c.pdf.
22. Faculdade de Medicina de São José do Rio Preto. Comitê de Ética em Pesquisa. [Internet]. [cited 2007 Oct 26]. Available from: http://www.cep.famerp.br/
23. Chalkley HW. Method for the quantitative morphologic analysis of tissues. J Natl Cancer Inst. 1943;4:47-53.
24. Shi SR, Key ME, Kalra KL. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem. 1991;39(6):741-8.
25. Ohara M, Sotozono C, Tsuchihashi Y, Kinoshita S. Ki-67 labeling index as a marker of malignancy in ocular surface neoplasms. Jpn J Ophthalmol. 2004; 48(6):524-9.
26. Ebato B, Friend J, Thoft RA. Comparison of limbal and peripheral human corneal epithelium in tissue culture. Invest Ophthalmol Vis Sci. 1988; 29(10):1533-7.
27. Gebhardt M, Mentlein R, Schaudig U, Pufe T, Recker K, Nölle B, et al. Differential expression of vascular endothelial growth factor implies the limbal origin of pterygia. Ophthalmology. 2005;112(6):1023-30.
28. Chowers I, Pe'er J, Zamir E, Livni N, Ilsar M, Frucht-Pery J. Proliferative activity and p53 expression in primary and recurrent pterygia. Ophthalmology. 2001;108(5):985-8.
29. Starck T, Kenyon KR, Serrano F. Conjunctival autograft for primary and recurrent pterygia: surgical technique and problem management. Cornea. 1991; 10(3):196-202.
30. Allan BD, Short P, Crawford GJ, Barrett GD, Constable IJ. Pterygium excision with conjunctival autografting: an effective and safe technique. Br J Ophthalmol. 1993;77(11):698-701. Comment in: Br J Ophthalmol. 1994;78(5):421.
31. Chen PP, Ariyasu RG, Kaza V, LaBree LD, McDonnell PJ. A randomized trial comparing mitomycin C and conjunctival autograft after excision of primary pterygium. Am J Ophthalmol. 1995;120(2):151-60.
32. Samahá JT, Schellini SA, Sakamoto RH, Padovani CR. Tratamento do pterígio recidivado por transplante autólogo de conjuntiva. Arq Bras Oftalmol. 2002; 65(4):415-8.
33. Cunha M, Alleman N. Transplante autólogo de conjuntiva no tratamento de pterígio primário e recidivado. Arq Bras Oftalmol. 1993;56(2):78-81.
34. Raiskup F, Solomon A, Landau D, Ilsar M, Frucht-Pery J. Mitomycin C for pterygium: long term evaluation. Br J Ophthalmol. 2004;88(11):1425-8.
35. Rubinfeld RS, Pfister RR, Stein RM, Foster CS, Martin NF, Stoleru S, et al. Serious complications of topical mitomycin-C after pterygium surgery. Ophthalmology. 1992;99(11):1647-54. Comment in: Ophthalmology. 1992;99(11): 1645-6. Ophthalmology. 1993;100(7):976-7; author reply 977-8. Ophthalmology. 1993;100(7):976; author reply 977-8. Ophthalmology. 1993;100(3):292-3.
36. Sugar A. Who should receive mitomycin-C after pterygium surgery? Ophthalmology. 1992;99(11):1645-6. Comment in: Ophthalmology. 1993;100(7): 976-7; author reply 977-8. Ophthalmology. 1993;100(7):976; author reply 977-8. Comment on: Ophthalmology. 1992;99(11):1647-54.
37. Dogru M, Erturk H, Shimazaki J, Tsubota K, Gul M. Tear function and ocular surface changes with topical mitomycin (MMC) treatment for primary corneal intraepithelial neoplasia. Cornea. 2003;22(7):627-39.
38. McKelvie PA, Daniell M. Impression cytology following mitomycin C therapy for ocular surface squamous neoplasia. Br J Ophthalmol. 2001;85(9):1115-9.
39. Di Girolamo N, Wakefield D, Coroneo MT. Differential expression of matrix metalloproteinases and their tissue inhibitors at the advancing pterygium head. Invest Ophthalmol Vis Sci. 2000;41(13):4142-9.
40. Leask A, Holmes A, Abraham DJ. Connective tissue growth factor: a new and important player in the pathogenesis of fibrosis. Curr Rheumatol Rep. 2002;4(2):136-42.
41. Kria L, Ohira A, Amemiya T. Immunohistochemical localization of basic fibroblast growth factor, platelet derived growth factor, transforming growth factor-beta and tumor necrosis factor-alpha in the pterygium. Acta Histochem. 1996;98(2):195-201.
42. Touhami A, Di Pascuale MA, Kawatika T, Del Valle M, Rosa RH Jr, Dubovy S, Tseng SC. Characterisation of myofibroblasts in fibrovascular tissues of primary and recurrent pterygia. Br J Ophthalmol. 2005;89(3):269-74.
43. Seifert P, Sekundo W. Capillaries in the epithelium of pterygium. Br J Ophthalmol. 1998;82(1):77-81.
44. Lelièvre SA, Weaver VM, Nickerson JA, Larabell CA, Bhaumik A, Petersen OW, Bissell MJ. Tissue phenotype depends on reciprocal interactions between the extracellular matrix and the structural organization of the nucleus. Proc Natl Acad Sci U S A. 1998;95(25):14711-6.
45. Kennedy M, Kim KH, Harten B, Brown J, Planck S, Meshul C, et al. Ultraviolet irradiation induces the production of multiple cytokines by human corneal cells. Invest Ophthalmol Vis Sci. 1997;38(12):2483-91.
46. Borden P, Heller RA. Transcriptional control of matrix metalloproteinases and the tissue inhibitors of matrix metalloproteinases. Crit Rev Eukaryot Gene Expr. 1997;7(1-2):159-78.
 Endereço para correspondência:
Endereço para correspondência:
Gildasio Castello de Almeida Junior
FAMERP - Av. Brigadeiro Faria Lima, 5.416
São José do Rio Preto (SP) CEP 15090-000
E-mail: [email protected]
Recebido para publicação em 23.04.2007
Versão recebida em 27.02.2008
Aprovação em 14.03.2008
Suporte Financeiro da Fundação de Amparo e Pesquisa (FAEPE - FAMERP) - São José do Rio Preto (SP) - Brasil.
Os Autores não tem nenhum interesse comercial ou financeiro em qualquer material ou equipamento citado nesse estudo.
Nota Editorial: Depois de concluída a análise do artigo sob sigilo editorial e com a anuência do Dr. José Álvaro Pereira Gomes sobre a divulgação de seu nome como revisor, agradecemos sua participação neste processo.
Trabalho realizado no Departamento de Oftalmologia da Faculdade de Medicina de São José do Rio Preto - FAMERP - São José do Rio Preto (SP) - Brasil.