

Amanda Pires Barbosa1; Monica Alves2; João Marcello Fortes Furtado1; Leidiane Adriano1; Luis Fernando Nominato1; Lara Cristina Dias1; Marina Zilio Fantucci1; Adriana de Andrade Batista Murashima1; Eduardo Melani Rocha1
DOI: 10.5935/0004-2749.20200102
ABSTRACT
The burden of corneal blindness and visual deficiency can be felt worldwide. Its association with several endemic diseases such as childhood blindness, trauma, infectious keratitis (including variants caused by herpes, hanseniasis, and fungi), vitamin A deficiency, diabetes mellitus, and other dry eye syndromes reflects its poorly understood underlying mechanisms and suggests that the actual frequency of the disease is underestimated. The low effectiveness of preventive and therapeutic strategies against corneal scarring or deformity predicts a high frequency of patients with corneal blindness in the future. Corneal blindness is associated with environmental factors and socioeconomic limitations that restrain health assistance and maintain a modest efficiency of the current therapeutic strategies for resolving corneal diseases in large-scale programs. We present here a critical review of the concepts associated with corneal blindness that need to be considered when planning strategies to prevent and treat corneal blindness worldwide (to be able to leave Plato’s cave, where corneal blindness is encaged.
Keywords: Blindness/epidemiology; Blindness/prevent & control; Blindness/therapy; Corneal opacity
RESUMO
O problema da deficiência visual e da cegueira corneal abrange o mundo todo e corresponde à quarta causa de cegueira e deficiência visual, com acometimento estimado e mais de 16 milhões de pessoas. A associação com várias doenças endêmicas, como cegueira infantil, trauma, ceratites infecciosas (incluindo herpes, hanseníase e fungos), hipovitaminose A, diabetes mellitus e outras causas de síndromes de olho seco, indicam que a verdadeira frequência é subestimada e que os diferentes mecanismos são pouco conhecidos. A baixa eficácia na prevenção e tratamento da cicatriz e deformidade da córnea permite antecipar que a prevalência da cegueira corneal irá crescer no futuro. As razões para o aumento da cegueira corneal envolvem fatores ambientais, limitações socioeconômicas para ampliar a assistência à saúde e a modesta eficiência das estratégias terapêuticas para resolver o problema em grande escala. O presente trabalho traz uma revisão crítica dos conceitos associados à cegueira corneal. Essa análise é uma etapa necessária para preparar o caminho com o objetivo de deixar a caverna que encarcera a cegueira corneal, em analogia ao mito de Platão, e melhorar as estratégias para prevenir e tratar a cegueira corneal em escala mundial.
Descritores: Cegueira/epidemiologia; Cegueira/prevenção & controle; Cegueira/terapia; Opacidade da córnea
INTRODUCTION
After a rapid overview of the medical literature and lectures presented in clinical conferences about corneal diseases, opacity, and corneal blindness, one may arrive at four conclusions: a) corneal blindness is a rare and distant problem; b) the causes are predictable, and the events leading up to corneal blindness are preventable; c) most of the causes of corneal injury are treatable, and the blindness outcome is avoidable; d) therapeutic approaches are very effective and long lasting(1-3).
These optimistic conclusions persist because most of the publications addressing corneal diseases fail to mention combined frequencies of the causes of corneal blindness, the rate of success and long-term outcomes of the available treatments, and the limitations to such treatments in the less technologically advanced and most populated regions of the planet. In the following sections, we will argue that corneal blindness is not well defined, that the above conclusions are wrong, and that a reduction of the burden of corneal blindness will not be achieved in large segments of the population using the present strategies.
The analogy with Plato’s cave in this work is justified by the apparent scenario where knowledge about corneal blindness is “fixed,” which brings to mind the fictional condition described by Plato in approximately 380 B.C. in “The Republic.” In a dialogue between Socrates and his brother Glauco, Socrates described individuals captive in a cave from a very young age, whose only sources of information are noises and shadows projected onto the cave wall in front of them. Prevented from leaving or even looking back, they are unable to understand their situation, until one of them escapes and makes contact with the outside world for the first time. After returning to the cave, the fugitive reports his experience to his former cave-mates and offers to help them escape. However, the captive individuals are skeptical and refuse the opportunity to be free(4).
The journey to understand the causes, frequency, mechanisms, and treatments of corneal opacity and blindness is reminiscent of the allegory of Plato’s cave in several ways. In the corneal blindness cave, the four assumptions enunciated above (a-d) are fed and supported by information produced by “normal” science, as defined by Thomas S. Kuhn in his work addressing the structure of scientific revolutions(5). In the cave, corneal opacity is a minor issue, well addressed in terms of public health and therapeutic strategies, and most symptoms can be solved with the refinement of certain therapeutic and surgical strategies. However, as in Plato’s cave, new knowledge is opening opportunities to address the challenge of corneal blindness, and this new information, which redefines the limits of our understanding, is met with skepticism.
Our aims with this review are to show data on the prevalence and mechanisms of corneal blindness, to explain why the problem is not improving, and to highlight the frustrating limitations associated with current treatment modalities. In the final section, we will show the perspectives for future corneal blindness treatments. In continuing with the analogy of Plato’s cave, the concepts brought forth by researchers, who left the “normal” science on corneal blindness, have been received with doses of skepticism.
The burden of corneal blindness
The World Health Organization (WHO) recently redefined visual impairment as a visual acuity >0.5 (or 20/40) and blindness as >0.05 (or 20/400) in the better-seeing eye, using the concept of “presented” instead of “best-corrected” visual acuity. This classification does not distinguish treatable from untreatable blindness or functional low vision(6,7).
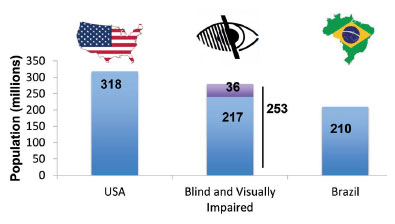
We can illustrate the difference between a blind eye and a blind person by referring to two portraits painted by Pablo Picasso in 1903. “Celestina” has an opaque (left) cornea but a normal right eye. In “The Blind Man’s Meal,” the character is using his hands to identify the food, and the face lacks the globe of the eye. These paintings illustrate the distinction between a blind person (“The Blind Man’s Meal”) according to the WHO definition and a person with a blind eye (Figure 1).

From recent estimates, the number of blind individuals in the world is approximately 36 million, and the number of those with moderate to severe visual deficiencies is 217 million(8). Taken together, this is a population comparable in size to those of the largest countries such as Brazil (211 million people) and the USA (327 million people)(8-10) (Figure 2).
The most frequent causes of visual impairment and blindness are uncorrected refractive errors and cataracts. Retinal diseases (including diabetic retinopathy), corneal blindness (summing trauma, infection, childhood blindness, vitamin A deficiency, and trachoma), and glaucoma present similar numbers of affected individuals(9,11). Glaucoma and retinal diseases have been addressed with technological improvements allowing early diagnosis and options for treatment that has reduced their prevalence among the causes of blindness during the past few decades(12-16). Data collection about blindness prevalence in population studies is oriented towards easily treatable causes in situations where two or more conditions contribute similarly to the blindness or visual impairment diagnosis(17).
Cataracts and refractive errors persist as large causes of visual impairment and blindness in studies and projections due to barriers against accessing eye health facilities and making technologies available in areas distant from big cities(18-21). Models of rapid interventions that leave communities without an established service have clearly failed to prevent or reduce visual impairment because of cataracts and refractive errors(22).
Grouped causes of corneal blindness and visual impairment may amount to a total of 16 million affected people, but the real numbers are difficult to obtain because of differences in search methods, regions evaluated, grouping, and analyses, as well as the uncertainty intervals of the estimated rates(9,11). A study in the Amazon region of Brazil revealed that pterygium, combined with corneal opacity, accounted for 12% of cases of blindness. Another study in Latin America showed corneal opacities as responsible for 4% of the cases of blindness, in contrast to the results of another study in São Paulo, Brazil, where corneal opacity and pterygium were not identified as causes of blindness(7,23-27).
Infectious keratitis (caused by bacteria, fungi, viruses, or parasites) can cause corneal opacities and blindness. However, the frequency of bilateral cases that would fit the definition of visual impairment and blindness has not been comprehensively registered, and the global prevalence remains unknown. Studies have suggested that infectious keratitis is the third- or fourth-leading cause of corneal blindness, behind pterygium, trauma, and surgery(28). Other frequent causes of corneal blindness identified in referral clinics and tertiary hospitals such as keratoconus and dysgenetic and dystrophic diseases (Peters’ anomaly, sclerocornea, and endothelial dystrophies) are underrepresented in population studies because they may be included in different groups as corneal opacities, refractive errors (keratoconus in the astigmatic group), and childhood blindness, and a systematic criteria to allow merging data from different epidemiological studies is lacking. Beyond this issue, an under-registered number of individuals with monocular visual impairment or binocular asymmetric corneal disease also exist. These observations indicate that more individuals will progress to corneal blindness in the future, and the numbers will also grow due to better registration techniques(8,26). The incidence of trachoma has shown a considerable decline in recent years, credited to the SAFE (surgery for trichiasis, antibiotics against Chlamydia, facial cleaning, and environmental improvements) strategy(14,29). In addition, other risk factors for corneal blindness and visual impairment tend to grow in the future because of factors, including increased life spans, limited access to treatments, and underestimated causes related to dry eye disease (DED): pterygium, pollutants, excessive ultraviolet light exposure, and the presence of high amounts of toxic agents in the environment(7,9,14,18,26-31).
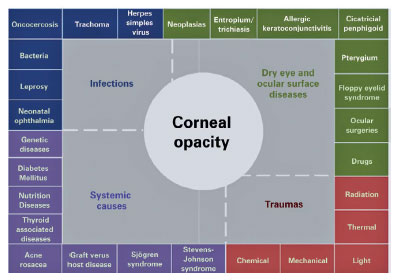
DED is a frequent and increasingly prevalent condition in the population(32) that is an underestimated risk factor for corneal blindness and is frequently associated with worse outcomes in diseases resulting in corneal blindness(33,34). Corneal opacities and consequent blindness may occur in diseases that cause DED (Figure 3). Vitamin A deficiency and trachoma are among the conditions that, when combined, lead to DED and corneal blindness(26,35). The prevalence of DED ranges between 5% and 50%, depending on diagnostic and inclusion criteria and geographic regions(32). The comorbidity and pathologic correlations between DED and corneal blindness deserve clarification. Moreover, DED causes, as potential risk factors for corneal blindness, need further studies.
Knowing the causes and managing the consequences
Could the rates of corneal blindness due to opacity be reduced if we knew its risk factors and demographics? Intuition and epidemiology say yes; that is what is observed in the epidemiologic triangle elaborated in the XIX century and its modern variations, which summarize the quote: “to know, to prevent”(36,37). Studies on the demographics of an individual with eye trauma over three decades have found practical difficulties: Patients that attend the clinic for eye trauma seem to be all young men with similar risk factors. In other words, observations from the 1980s to the 2000s have revealed that young, male handworkers are more likely to suffer eye traumas (frequently wounding the cornea), mostly in the workplace, leading to similar functional, social, and economic consequences in all of them(38). In that report, approximately 80% of patients were not using protective devices, and 30% had had at least another ocular trauma. These data are similar in other regions throughout the world(16,28,39,40). The same observations can be extrapolated to infectious keratitis, especially that caused by filamentary fungi, a devastating corneal infection that occurs mostly in young, male agricultural workers, and which presents few therapeutic options and carries poor prognoses for the cornea and vision(41-45). Ibrahim et al. found that fungal keratitis has a seasonal prevalence associated with low humidity and low temperatures; approximately 38% of the cases resulted in therapeutic corneal transplants (15% with eye globe evisceration), and approximately 60% have a blind eye by the first year of follow-up(42,44). Less common but also strongly associated with a specific risk factor is Acanthamoeba keratitis, where the association is with bad compliance with proper contact lens care(46-48).
These observations indicate that preventive measures can be used to reduce the occurrence of ocular trauma and infectious keratitis, two major causes of corneal blindness(26,28) (Table 1). However, the frequency of these illnesses and the same patient profiles can be found throughout the world. Therefore, the myth of Plato’s cave applies to corneal blindness, revealing the shadows on the wall to be topics on the size of the problem and its “accidental” causes. Two of the previous assumptions can be refuted by data: corneal blindness is not rare, and despite knowledge on its epidemiology, the incidence remains high.
Established and useful concepts on corneal structure and physiology
The cornea is a transparent organ that fills 1 cm2 of the area in front of the eyeball. It has a hemispherical format and less than 1 mm in thickness. It is almost 90% transparent and mostly composed of water and acts as a shield for the eye globe. Given its fragile profile, a major challenge is to understand how the cornea resists and responds to environmental and external aggressions(56).
The surface is protected by a tear film with a complex and variable composition(57). Its ingredients are produced in the exocrine glands present in the ocular surface and the goblet and epithelial cells in the epithelial layer. The tear film flows as a result of the eyelids blinking at an average pace of 10 to 20 times per minute, which renews the tear film volume of 10 μL at a rate of 1 μL/ minute(57). This mechanism allows for nutrition, protection, and stimuli to the cells of the eye that are replaced throughout the life of an individual(57,58).
The five layers of the cornea have well-described roles that allow it to act as a barrier for the whole eye and as an efficient optical lens. The corneal epithelium prevents microorganism and toxic agent invasions, the endothelium controls the water content, and the stroma gives transparency and dioptric power to match the needs of the eye. The stroma is found between two membranes: Bowman’s membrane, on the external side of the cornea, separates the stroma from the epithelial-layer basement membrane, and Descemet’s membrane, on the internal side of the cornea, separates the stroma from the endothelium(59). Improvements and growth in the number of lamellar corneal transplants have drawn the attention of cornea surgeons allowing for the characterization of a pre-Descemet membrane, also called Dua’s membrane(60), a more compact corneal layer, with few keratocytes found between Descemet’s membrane and the posterior part of the stroma. After some skepticism, this sixth layer is being gradually accepted, and it has been found to be associated with the mechanism of corneal hydrops and the elastic resistance of the descemetocele, at the same time being used as a safety variable during surgical techniques for deep anterior lamellar keratoplasty (a form of corneal transplant)(61,62).
The transparency of the cornea is supported mainly by its avascularity. Blood and lymphatic vessels grow in the cornea from the corneal limbus in response to aggressions and inflammation(63). The avascularity is sustained by the permanent expression of soluble vascular endothelial growth factor receptor (sVEGFR) in the ocular surface and in the stroma(64). When sVEGFR is suppressed, new vessels grow in the cornea(64).
A dense network of nerve fibers detects external, harmful stimuli and modulates the reactions of the cornea(65-67). These nerve fibers are linked to the environment by a family of transient receptor potential (TRP) channels activated by environmental variations in temperature, pH, osmolarity, and mechanical stimuli(68). The responses escalate based on the intensity of the stimulus and trigger signals capable of attracting inflammatory mediators that activate wound healing processes(69,70). Interestingly, aggressions limited to the epithelium are relatively benign, and the body is able to restore the epithelial homeostasis a short time after the initial offense; however, aggressions that hit the stroma leave long lasting or even permanent scars in the stromal layer(71). Two recently described mechanisms help to explain this response: The first one involves activation of the transient receptor potential vanilloid 1 (TRPV1) in epithelial cell cultures by osmolarity, temperature, and chemical challenges, which induce secretion of inflammatory cytokines (IL-6 and IL-8) through the Mitogen-Activated Protein Kinase signaling pathway, but also induces corneal epithelial cell migration through epidermal growth factor receptor transactivation(72-75). In addition, the activation of TRPV channels in keratocytes present in a deeper corneal layer (the stroma) promotes the secretion of transforming growth factor-beta and induces the production of collagen, which is responsible for stromal scar formation(70,76-79). Taken together, these findings indicate that superficial damage to the epithelium prompts fast wound healing and the preservation of transparency despite a painful and inflammatory process and deeper injuries to the corneal stroma, which destroys the nerve network and jeopardizes the eye globe integrity or triggers a mechanism of new vessel growth, and strengthens the globe wall (corneal stroma). The delicate structure that provides transparency is abdicated in favor of a scar, which is an opaque and stronger barrier against external injuries. To attend that natural rule of corneal transparency, mechanisms are in place to actively and wisely protect the cornea against neovascularization, where dense innervation not only provides high sensitivity but also inhibits neovascularization; however, the opposite occurs in response to corneal damage, denervation, or nerve network damage that allows for neovascularization, which, in turn, inhibits reinnervation(80).
Connections among the corneal layers also respond to persistent injuries. One example is the chronic use of contact lenses, which leads to changes in the shape of endothelial cells; the other example is bullous keratopathy(81,82), which induces stromal edema due to a lack of deturgescence control resulting from the loss of endothelial cells and from inflammatory events in the ocular surface that induce neovascularization and loss of limbal stem and goblet cells(81). In this disease, the repercussions to the stroma and ocular surface may be explained by endothelial cell responses to a hypotonic environment that induce a paracrine secretion of inflammatory cytokines, mediated also by the TRPV1 channels(78,83,84).
Taken together, this information helps to clarify the initial mediators and steps in the mechanisms underlying the fast superficial lesion repairs without inflammation. On the other hand, the lesions that hit the stroma or the endothelium, either from the external or internal side of the eye and whose effects last for a long period of time, can induce extensive inflammatory processes and a permanent corneal scar.
Epithelial replacement is crucial for faster wound healing, and corneal epithelial stem cells asymmetrically distributed in niches on the limbal region, called the palisades of Vogt, prevent the development of lesions to the stromal layer(85-87). During the last three decades, explanations have been provided for how these stem cells renew and replace corneal basal epithelial cells(58,88). What is not clear is the manner in which epithelial wound healing occurs independently of the corneal limbal epithelial cells in certain animal eye lesion models and clinical conditions(89-93).
In summary, the five recent observations about the corneal structure and the response to injuries are examples of relevant information brought to the Plato’s cave of corneal blindness that, once overcoming the initial wave of skepticism and being applied to treatments for corneal blindness, will change the epidemiological scenario described above (Table 2). Interestingly, a review article authored by Tseng and Tsubota in 1997 advanced some of these concepts, although without the steps or molecular details(58).
History and present limitations of using penetrating keratoplasty for the treatment of corneal opacity
The major strategy for fixing an opaque, perforated, or melted cornea is a replacement of the organ. Conceptualizations and improvements in the technique have been described in other reviews with minimum variations in terms of historical details(94-96). The first physician credited with mentioning the possibility of corneal replacement was Galen, in Greece, sometime between 130 and 200 A.D. In the XVIII century, different authors conceptualized the possibility of curing corneal blindness. Among them were Erasmus Darwin and Guillaume Pellier de Quengsy(94-96). During the XIX century, a heterologous strategy (i.e., using the cornea from other animal species) was evaluated by several authors, and in the XX century, the first homologous transplant took place. The remarkable advances in the understanding of the biology of grafts, from the 1960 Nobel Prize awarded to Peter Medawar to the studies on mechanisms of immunotolerance, clarified the players involved in the success and failure of corneal transplantations(63,97). The penetrating keratoplasty technique improved considerably after the 1960s with the introduction of four key elements: eye banking, surgical microscopy, 10-0 nylon sutures, and post-operatory corticotherapy(94,98).
The limitations that impede the success of this procedure can be summarized by two points (Table 3):
1) The limited availability of corneas for all cases of corneal blindness. An average of 180,000 corneal transplants is performed every year worldwide, far less compared with the 16 million patients with corneal opacities and low vision or blindness(9,11,99,100). In fact, the estimated number of donor corneas or penetrating keratoplasties available every year covers only 1 out of every 70 cases awaiting this treatment(99). A considerable effort to increase the number of donor tissues and the number of facilities to treat those people would be necessary to revert corneal blindness with this strategy. Considering these numbers, the capacity must grow several times over to be able to meet the present demand.
2) The survivor curve of corneal transplants worsens the limitation of donor corneas. Studies have revealed that under favorable conditions, the half-life of a graft is approximately 12 years; however, in adverse situations such as massive inflammation (therapeutic) or perforated cornea (tectonic), the mean survival time of the grafts is as low as 5 and 2 years, respectively(101-103).
Insisting on the strategy of corneal transplants under these unfavorable conditions is an unwise option to revert corneal blindness(100,104-108). Since the 1970s, clinical scientists and researchers have worked together to develop alternatives or complementary strategies to corneal transplants(95,106). The most recent options, including pharmaceutical and surgical alternatives to avoid corneal blindness, will be addressed in a subsequent review, but lying beyond the shadows and the skepticism are useful and innovative strategies for treating corneal blindness, as supported by the concepts presented above.
This review summarized the problem of corneal blindness, addressing epidemiological flaws and the mechanisms of the major causes of this disease with the limitations of relying on corneal transplants to provide a cure and reduce the number of blindness conditions worldwide. We believe our review sheds a light on the shadows inside the cave and summarizes the work of researchers who, upon leaving the cave and observing beyond its opening, have returned with rich pieces of information for understanding the size of the problem, its detailed physiopathology, and the fragility of the present therapeutic strategies for treating corneal blindness.
ACKNOWLEDGMENTS
The authors thank the following Brazilian governmental institutions for their financial support in the form of grants: Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP) (nº 2015/20580-7 and 2014/22451-7) (São Paulo, SP, Brazil); Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (nº 474450/2012-0) (Brasilia, DF, Brazil); CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) (Finance Code 001) (Brasilia, DF, Brazil); Fundação de Apoio ao Ensino, Pesquisa e Assistência do Hospital das Clinicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo (FAEPA) (669/2018) (Ribeirão Preto, SP, Brazil); and the Research Core of Ocular Physiopathology and Therapeutics from University of São Paulo (NAP-FTO) (nº 12.1.25431.01.7) (Ribeirão Preto, SP, Brazil).
REFERENCES
1. Krachmer J, Mannis MJ, Holland EC. Cornea. 3rd ed. China: Mosby Elsevier; 2010. p. 1920.
2. Jaeger EA, Tasman W. Duane’s ophthalmology: Lippincolt Williams & Wilkinns; 2013.
3. Albert DM, Jakobiec FA, Miller JW, Azar DT. Albert & Jakobiec’s principles and practice of ophthalmology. Elsevier Saunders; 2008.
4. Platão. A República: ou sobre a justiça e diálogo político/The Republic: or on justice and political dialogue. 2a ed. São Paulo, Brasil: Martins Fontes; 2006. p.419.
5. Kuhn TS. The structure of the scientific revolutions. 2nd ed. Chicago: University of Chicago; 1970.
6. World Health Organization (WHO). Blindness and vision impairment. Geneva: WHO; 2018.
7. Limburg H, Espinoza R, Lansingh VC, Silva JC. Functional low vision in adults from Latin America: findings from population-based surveys in 15 countries. Rev Panam Salud Publica. 2015;37(6):371-8.
8. Bourne RR, Flaxman SR, Braithwaite T, Cicinelli MV, Das A, Jonas JB, et al.; Vision Loss Expert Group. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(9):e888-97.
9. Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96(5):614-8.
10. Worldometers [Internet]. Current world population. [cited 2019 Dec 26]. Available from: http://www.worldometers.info/world-population/.
11. Flaxman SR, Bourne RR, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV, et al.; Vision Loss Expert Group of the Global Burden of Disease Study. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(12):e1221-34.
12. Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S. Glaucoma. Lancet. 2017;390(10108):2183-93.
13. Realini T, Fechtner RD. 56,000 ways to treat glaucoma. Ophthalmology. 2002;109(11):1955-6.
14. Resnikoff S, Keys TU. Future trends in global blindness. Indian J Ophthalmol. 2012;60(5):387-95.
15. Bajwa A, Aman R, Reddy AK. A comprehensive review of diagnostic imaging technologies to evaluate the retina and the optic disk. Int Ophthalmol. 2015;35(5):733-55.
16. Liew G, Wong VW, Ho IV. Mini Review: Changes in the Incidence of and Progression to Proliferative and Sight-Threatening Diabetic Retinopathy Over the Last 30 Years. Ophthalmic Epidemiol. 2017; 24(2):73-80.
17. Kuper H, Polack S, Limburg H. Rapid assessment of avoidable blindness. Community Eye Health. 2006;19(60):68-9.
18. Balarabe AH, Mahmoud AO, Ayanniyi AA. The Sokoto blind beggars: causes of blindness and barriers to rehabilitation services. Middle East Afr J Ophthalmol. 2014;21(2):147-52.
19. Burga HG, Hinds CN, Lansingh VC, Samudio M, Lewallen S, Courtright P, et al. Is the cost the primary barrier for cataract surgery in Paraguay? Arq Bras Oftalmol. 2014;77(3):164-7.
20. Melese M, Alemayehu W, Friedlander E, Courtright P. Indirect costs associated with accessing eye care services as a barrier to service use in Ethiopia. Trop Med Int Health. 2004;9(3):426-31.
21. Hong H, Mújica OJ, Anaya J, Lansingh VC, López E, Silva JC. The Challenge of Universal Eye Health in Latin America: distributive inequality of ophthalmologists in 14 countries. BMJ Open. 2016; 6(11):e012819.
22. De Senne FM, Cardillo JA, Rocha EM, Kara-José N. Long-term visual outcomes in the Cataract-Free Zone Project in Brazil. Acta Ophthalmol Scand. 2002;80(3):262-6.
23. Salomao SR, Cinoto RW, Berezovsky A, Araujo-Filho A, Mitsuhiro MR, Mendieta L, et al. Prevalence and causes of vision impairment and blindness in older adults in Brazil: the Sao Paulo Eye Study. Ophthalmic Epidemiol. 2008;15(3):167-75.
24. Furtado JM, Berezovsky A, Ferraz NN, Muñoz S, Fernandes AG, Watanabe SS, et al. Prevalence and causes of visual impairment and blindness in adults aged 45 years and older from parintins: The Brazilian Amazon Region Eye Survey. Ophthalmic Epidemiol. 2019;26(5):345-54.
25. Chávez GM, de Barrios AR, Pojoy OL, de Reyes AR, Melgar MY, Melgar JF, et al. National survey of blindness and visual impairment in Guatemala, 2015. Arq Bras Oftalmol. 2019;82(2):91-7.
26. Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79(3):214-21.
27. Fernandes AG, Salomão SR, Ferraz NN, Mitsuhiro MH, Furtado JM, Muñoz S, et al. Pterygium in adults from the Brazilian Amazon Region: prevalence, visual status and refractive errors. Br J Ophthalmol. 2019 Sep 18;bjophthalmol-2019-314131. doi: 10.1136/bjophthalmol-2019-314131. [Epub ahead of print]. PMID: 31533928.
28. Gupta N, Vashist P, Tandon R, Gupta SK, Dwivedi S, Mani K. Prevalence of corneal diseases in the rural Indian population: the Corneal Opacity Rural Epidemiological (CORE) study. Br J Ophthalmol. 2015;99(2):147-52.
29. Taylor HR, Burton MJ, Haddad D, West S, Wright H. Trachoma. Lancet. 2014;384(9960):2142-52. 30.
30. Pontelli RC, Souza MC, Fantucci MZ, de Andrade M, Rocha EM. The role of endocrine disruptors in ocular surface diseases. Med Hypotheses. 2019;122:157-64.
31. Torricelli AA, Novaes P, Matsuda M, Alves MR, Monteiro ML. Ocular surface adverse effects of ambient levels of air pollution. Arq Bras Oftalmol. 2011;74(5):377-81.
32. Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, et al. TFOS DEWS II Epidemiology report. Ocul Surf. 2017;15(3):334-65.
33. Pflugfelder SC. Tear dysfunction and the cornea: LXVIII Edward Jackson Memorial Lecture. Am J Ophthalmol. 2011;152:900-909 e901.
34. Coster DJ. Fundamentals of Clinical Ophthalmology: Cornea. In: Lightman S, editor. Fundamentals of clinical ophthalmology. London: BMJ Books; 2002. p. 1-34.
35. Lemp MA. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. CLAO J. 1995;21(4):221-32.
36. 3Renton A. Epidemiology and causation: a realist view. J Epidemiol Community Health. 1994;48(1):79-85.
37. Rothman KJ. Causes. Am J Epidemiol. 1976;104(6):587-92.
38. Alves Cecchetti DF, de Paula Cecchetti SA, Tremeschini Nardy AC, Carvalho SC. Veronese Rodrigues ML, Rocha EM. A clinical and epidemiological profile of ocular emergences in a reference emergency center. Arq Bras Oftalmol. 2008;71:635-8.
39. May DR, Kuhn FP, Morris RE, Witherspoon CD, Danis RP, Matthews GP, et al. The epidemiology of serious eye injuries from the United States Eye Injury Registry. Graefes Arch Clin Exp Ophthalmol. 2000;238(2):153-7.
40. Al-Mahrouqi HH, Al-Harthi N, Al-Wahaibi M, Hanumantharayappa K. Ocular trauma: A tertiary hospital experience from Oman. Oman J Ophthalmol. 2017;10(2):63-9.
41. Ibrahim MM, Vanini R, Ibrahim FM, Fioriti LS, Furlan EM, Provinzano LM, et al. Epidemiologic aspects and clinical outcome of fungal keratitis in southeastern Brazil. Eur J Ophthalmol. 2009; 19(3):355-61. 42.
42. Ibrahim MM, Vanini R, Ibrahim FM, Martins WP, Carvalho RT, Castro RS, et al. Epidemiology and medical prediction of microbial keratitis in southeast Brazil. Arq Bras Oftalmol. 2011;74(1):7-12.
43. Ibrahim MM, de Angelis R, Lima AS, Viana de Carvalho GD, Ibrahim FM, Malki LT, et al. A new method to predict the epidemiology of fungal keratitis by monitoring the sales distribution of antifungal eye drops in Brazil. PLoS One. 2012;7(3):e33775.
44. Tuft SJ, Tullo AB. Fungal keratitis in the United Kingdom 2003- 2005. Eye (Lond). 2009;23(6):1308-13.
45. Wang H, Zhang Y, Li Z, Wang T, Liu P. Prevalence and causes of corneal blindness. Clin Exp Ophthalmol. 2014;42(3):249-53.
46. Cope JR, Collier SA, Schein OD, Brown AC, Verani JR, Gallen R, et al. Acanthamoeba Keratitis among Rigid Gas Permeable Contact Lens Wearers in the United States, 2005 through 2011. Ophthalmology. 2016;123(7):1435-41.
47. Truong DT, Bui MT, Memon P, Cavanagh HD. Microbial Keratitis at an Urban Public Hospital: A 10-Year Update. J Clin Exp Ophthalmol. 2015;6(6):6.
48. Marujo FI, Hirai FE, Yu MC, Hofling-Lima AL, Freitas D, Sato EH. [Distribution of infectious keratitis in a tertiary hospital in Brazil]. Arq Bras Oftalmol. 2013;76(6):370-3.
49. Rao SK, Greenberg PB, Filippopoulos T, Scott IU, Katsoulakis NP, Enzer YR. Potential impact of seatbelt use on the spectrum of ocular injuries and visual acuity outcomes after motor vehicle accidents with airbag deployment. Ophthalmology. 2008;115:573-6 e1.
50. Tseng VL, Linakis JG, Mello MJ, Greenberg PB. Patterns of ocular injury from paintball trauma. Eye (Lond). 2014;28(10):1266-7.
51. Recommendations for the prevention of neonatal ophthalmia. Paediatr Child Health. 2002;7(7):480-8.
52. Kuhn F. Ocular traumatology: prevention, prevention, prevention.... Graefes Arch Clin Exp Ophthalmol. 2010;248(3):299-300.
53. Oliva MS, Schottman T, Gulati M. Turning the tide of corneal blindness. Indian J Ophthalmol. 2012;60(5):423-7.
54. Kim YE, Remme JH, Steinmann P, Stolk WA, Roungou JB, Tediosi F. Control, elimination, and eradication of river blindness: scenarios, timelines, and ivermectin treatment needs in Africa. PLoS Negl Trop Dis. 2015;9(4):e0003664.
55. Travers A, Strasser S, Palmer SL, Stauber C. The added value of water, sanitation, and hygiene interventions to mass drug administration for reducing the prevalence of trachoma: a systematic review examining. J Environ Public Health. 2013;2013:682093.
56. Pepose JS, Ubels JL. The cornea. In: Hart WM Jr, editor. Adler’s physiology of the eye. St. Louis: Mosby Year Book; 1992. p. 29-70.
57. Lemp MA, Wolfley DE. The lacrimal apparatus. In: Hart WM Jr, editor. Adler’s physiology of the eye. St. Louis: MosbyYear Book, Inc.; 1992. p. 18-28.
58. Tseng SC, Tsubota K. Important concepts for treating ocular surface and tear disorders. Am J Ophthalmol. 1997;124(6):825-35.
59. Dawson DG, Watsky MA, Geroski DH, Edelhouser HF. Cornea and sclera. In: Tasman W, Jaeger EA, editors. Duane’s ophthalmology. Philadelphia: Lippincott Willians and Wilkins; 2008. p. 75.
60. Dua HS, Faraj LA, Said DG, Gray T, Lowe J. Human corneal anatomy redefined: a novel pre-Descemet’s layer (Dua’s layer). Ophthalmology. 2013;120(9):1778-85.
61. Dua HS, Said DG. Clinical evidence of the pre-Descemets layer (Dua’s layer) in corneal pathology. Eye (Lond). 2016;30(8):1144-5.
62. Yahia Chérif H, Gueudry J, Afriat M, Delcampe A, Attal P, Gross H, et al. Efficacy and safety of pre-Descemet’s membrane sutures for the management of acute corneal hydrops in keratoconus. Br J Ophthalmol. 2015;99(6):773-7.
63. Cursiefen C, Chen L, Dana MR, Streilein JW. Corneal lymphangiogenesis: evidence, mechanisms, and implications for corneal transplant immunology. Cornea. 2003;22(3):273-81.
64. Ambati BK, Nozaki M, Singh N, Takeda A, Jani PD, Suthar T, et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443(7114):993-7.
65. Belmonte C, Acosta MC, Gallar J. Neural basis of sensation in intact and injured corneas. Exp Eye Res. 2004;78(3):513-25.
66. Bonini S, Rama P, Olzi D, Lambiase A. Neurotrophic keratitis. Eye (Lond). 2003;17(8):989-95.
67. Marfurt CF, Cox J, Deek S, Dvorscak L. Anatomy of the human corneal innervation. Exp Eye Res. 2010;90(4):478-92.
68. Pan Z, Yang H, Reinach PS. Transient receptor potential (TRP) gene superfamily encoding cation channels. Hum Genomics. 2011; 5(2):108-16.
69. Hiura A. Is thermal nociception only sensed by the capsaicin receptor, TRPV1? Anat Sci Int. 2009;84(3):122-8.
70. Okada Y, Reinach PS, Shirai K, Kitano A, Kao WW, Flanders KC, et al. TRPV1 involvement in inflammatory tissue fibrosis in mice. Am J Pathol. 2011;178(6):2654-64.
71. Obata H, Tsuru T. Corneal wound healing from the perspective of keratoplasty specimens with special reference to the function of the Bowman layer and Descemet membrane. Cornea. 2007;26(9 Suppl 1):S82-9.
72. Yang H, Wang Z, Capó-Aponte JE, Zhang F, Pan Z, Reinach PS. Epidermal growth factor receptor transactivation by the cannabinoid receptor (CB1) and transient receptor potential vanilloid 1 (TRPV1) induces differential responses in corneal epithelial cells. Exp Eye Res. 2010;91(3):462-71.
73. Mergler S, Garreis F, Sahlmüller M, Reinach PS, Paulsen F, Pleyer U. Thermosensitive transient receptor potential channels in human corneal epithelial cells. J Cell Physiol. 2011;226(7):1828-42.
74. Zhang F, Yang H, Wang Z, Mergler S, Liu H, Kawakita T, et al. Transient receptor potential vanilloid 1 activation induces inflammatory cytokine release in corneal epithelium through MAPK signaling. J Cell Physiol. 2007;213(3):730-9.
75. Pan Z, Wang Z, Yang H, Zhang F, Reinach PS. TRPV1 activation is required for hypertonicity-stimulated inflammatory cytokine release in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2011;52(1):485-93.
76. Okada Y, Reinach PS, Shirai K, Kitano-Izutani A, Miyajima M, Yamanaka O, et al. Transient receptor potential channels and corneal stromal inflammation. Cornea. 2015;34 Suppl 11:S136-41.
77. Tomoyose K, Okada Y, Sumioka T, Miyajima M, Flanders KC, Shirai K, et al. Suppression of in vivo neovascularization by the loss of TRPV1 in mouse cornea. J Ophthalmol. 2015;2015:706404.
78. Mergler S, Valtink M, Takayoshi S, Okada Y, Miyajima M, Saika S, et al. Temperature-sensitive transient receptor potential channels in corneal tissue layers and cells. Ophthalmic Res. 2014;52(3):151-9.
79. Okada Y, Shirai K, Miyajima M, Reinach PS, Yamanaka O, Sumioka T, et al. Loss of TRPV4 function suppresses inflammatory fibrosis induced by Alkali-Burning mouse Corneas. PLoS One. 2016; 11(12):e0167200.
80. Ferrari G, Hajrasouliha AR, Sadrai Z, Ueno H, Chauhan SK, Dana R. Nerves and neovessels inhibit each other in the cornea. Invest Ophthalmol Vis Sci. 2013;54(1):813-20.
81. Uchino Y, Goto E, Takano Y, Dogru M, Shinozaki N, Shimmura S, et al. Long-standing bullous keratopathy is associated with peripheral conjunctivalization and limbal deficiency. Ophthalmology. 2006;113(7):1098-101.
82. Bourne WM. The effect of long-term contact lens wear on the cells of the cornea. CLAO J. 2001;27(4):225-30.
83. Mergler S, Valtink M, Coulson-Thomas VJ, Lindemann D, Reinach PS, Engelmann K, et al. TRPV channels mediate temperature-sensing in human corneal endothelial cells. Exp Eye Res. 2010;90(6):758-70.
84. Mergler S, Valtink M, Taetz K, Sahlmüller M, Fels G, Reinach PS, et al. Characterization of transient receptor potential vanilloid channel 4 (TRPV4) in human corneal endothelial cells. Exp Eye Res. 2011;93(5):710-9.
85. Yeung AM, Schlötzer-Schrehardt U, Kulkarni B, Tint NL, Hopkinson A, Dua HS. Limbal epithelial crypt: a model for corneal epithelial maintenance and novel limbal regional variations. Arch Ophthalmol. 2008;126(5):665-9.
86. Dua HS, Shanmuganathan VA, Powell-Richards AO, Tighe PJ, Joseph A. Limbal epithelial crypts: a novel anatomical structure and a putative limbal stem cell niche. Br J Ophthalmol. 2005;89(5):529-32.
87. Echevarria TJ, Di Girolamo N. Tissue-regenerating, vision-restoring corneal epithelial stem cells. Stem Cell Rev Rep. 2011;7(2):256-68.
88. Thoft RA, Friend J. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci. 1983;24(10):1442-3.
89. Góes RM, Barbosa FL, De Faria-E-Sousa SJ, Haddad A. Morphological and autoradiographic studies on the corneal and limbal epithelium of rabbits. Anat Rec (Hoboken). 2008;291(2):191-203.
90. Barbosa FL, Góes RM, de Faria-E-Sousa SJ, Haddad A. Regeneration of the corneal epithelium after debridement of its central region: an autoradiographic study on rabbits. Curr Eye Res. 2009;34(8):636-45.
91. Dua HS, Miri A, Alomar T, Yeung AM, Said DG. The role of limbal stem cells in corneal epithelial maintenance: testing the dogma. Ophthalmology. 2009;116(5):856-63.
92. Majo F, Rochat A, Nicolas M, Jaoudé GA, Barrandon Y. Oligopotent stem cells are distributed throughout the mammalian ocular surface. Nature. 2008;456(7219):250-4.
93. Sun TT, Tseng SC, Lavker RM. Location of corneal epithelial stem cells. Nature. 2010;463(7284):E10-1.
94. Moffatt SL, Cartwright VA, Stumpf TH. Centennial review of corneal transplantation. Clin Exp Ophthalmol. 2005;33(6):642-57. 95.
95. Crawford AZ, Patel DV, McGhee CN. A brief history of corneal transplantation: from ancient to modern. Oman J Ophthalmol. 2013;6(4 Suppl 1):S12-7.
96. Güell JL, El Husseiny MA, Manero F, Gris O, Elies D. Historical review and update of surgical treatment for corneal endothelial diseases. Ophthalmol Ther. 2014;3(1-2):1-15.
97. Niederkorn JY. Cornea: Window to Ocular Immunology. Curr Immunol Rev. 2011;7(3):328-35.
98. Fahd DS, Alleman N, Chamon W. History of Cornea Surgery. In: Copeland RA Jr, Afshari NA, editors. Principles and practice of cornea. New Delhi, India: Jaypee Medical Publishers Ltd.; 2013. p. 899-916.
99. Gain P, Jullienne R, He Z, Aldossary M, Acquart S, Cognasse F, et al. Global survey of corneal transplantation and eye banking. JAMA Ophthalmol. 2016;134(2):167-73.
100. Garg P, Krishna PV, Stratis AK, Gopinathan U. The value of corneal transplantation in reducing blindness. Eye (Lond). 2005;19(10):1106-14.
101. Tan DT, Janardhanan P, Zhou H, Chan YH, Htoon HM, Ang LP, Lim LS. Penetrating keratoplasty in Asian eyes: the Singapore Corneal Transplant Study. Ophthalmology. 2008;115(6):975-982.e1.
102. Coster DJ, Williams KA. The impact of corneal allograft rejection on the long-term outcome of corneal transplantation. Am J Ophthalmol. 2005;140(6):1112-22.
103. Dandona L, Naduvilath TJ, Janarthanan M, Ragu K, Rao GN. Survival analysis and visual outcome in a large series of corneal transplants in India. Br J Ophthalmol. 1997;81(9):726-31.
104. Kontis V, Bennett JE, Mathers CD, Li G, Foreman K, Ezzati M. Future life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. Lancet. 2017;389(10076):1323-35.
105. Al-Swailem SA. Graft failure: II. Ocular surface complications. Int Ophthalmol. 2008;28(3):175-89.
106. Coster DJ. Doyne Lecture. Influences on the development of corneal transplantation. Eye (Lond). 1994;8(Pt 1):1-11.
107. Coster DJ, Williams KA. The Australian Corneal Graft Registry (ACGR). Klin Monatsbl Augenheilkd. 1994;205(5):271-4.
108. Vieira Silva J, Júlio de Faria e Sousa S, Mafalda Ferrante A. Corneal transplantation in a developing country: problems associated with technology transfer from rich to poor societies. Acta Ophthalmol Scand. 2006;84(3):396-400.
Submitted for publication:
June 25, 2019.
Accepted for publication:
November 19, 2019.
Funding: This study received no specific financial support.
Disclosure of potential conflicts of interest: None of the authors have any potential conflicts of interest to disclose.