INTRODUCTION
Glaucoma has a pathophysiology that is very similar to that of hydrocephalus. Both diseases are characterized by a disturbance of the production and absorption of liquids. The function of the cerebrospinal fluid (CSF) and the aqueous humor and their secretion and absorption are remarkably similar.
Hydrocephalus is defined as a disorder of the physiology of CSF(1). The prevalence of hydrocephalus in the population is 1-2%(2-3). Under normal conditions, CSF is produced by the choroid plexus(1) at a rate of 500 ml/day. It is reabsorbed gradually by a passive process into the venous system via the arachnoid villi(4) (arachnoid granulation).
In total, 70,000 cases of hydrocephalus are annually admitted to hospitals in the United States; of these, between 18 and 33,000 will be given CSF shunt devices(3).
Glaucoma is the leading cause of irreversible blindness worldwide(5-6), with a prevalence of up to 4.74%(7). It is characterized by damage to the optic nerve fiber layer, resulting in progressive visual field disturbance. Intraocular pressure (IOP) is the most important risk factor for glaucoma; and currently, decreasing IOP is the only treatment option. Aqueous humor is produced by the ciliary body at a rate of 2.8-3.6 ml per day(8), circulating from the posterior chamber to the anterior chamber, and it is absorbed into the venous system through the trabecular meshwork.
The latest development in the treatment of hydrocephalus has been the introduction of programmable valves(9). These enable the desired pressure levels for each patient to be regulated in the doctor’s office, avoiding the complications of under- or over-drainage of CSF, without the need for further surgery to change the devices(10). Reviewing the current management of hydrocephalus, a system to shunt aqueous humor from the anterior chamber to the peritoneum has been developed. The surgical technique is a modification of the technique currently used by neurosurgeons to treat hydrocephalus, with the ventricular catheter being replaced by an ocular catheter.
CASE REPORT
The patient presented in this paper is enrolled in an ongoing research protocol at the Clínica de Ojos Maldonado Bas. The protocol has been approved by Oulton-Romagosa Institutional Health Research Ethics Committee, and respects the norms and standards enshrined in the Nuremberg Code, the Declaration of Helsinki, CIOMS/WHO Guidelines, the Universal Declaration on Bioethics and Human Rights (UNESCO), and other international and national instruments governing biomedical research.
A 40-year-old male patient with glaucoma presented to the clinic. He had a history of bilateral keratoplasty for keratoconus during adolescence. After the corneal surgery he had developed bilateral glaucoma. He had undergone cataract surgery and 2 filtering surgeries in the right eye (OD) and filtering surgery in the left eye (OS). The visual acuity was no light perception in the OD and 4/10 in the OS. Slit-lamp examination of the OD showed keratoplasty with epithelial edema, a non-reactive dilated pupil with sector iridectomy, capsular pseudoexfoliation, pseudophakia, upper scleral thinning, and 2 non-functioning filtering blebs. IOP was 50 mmHg. Slit-lamp examination of the OS showed keratoplasty, permeable iridectomy, a transparent lens, and a functioning filtering bleb. IOP was 11 mmHg.
An aqueous humor shunt to the peritoneum was performed in the OD. A hydrocephalus valve (Medtronic PS Medical® Strata® NSC) was used, regulated at level 2.5 (to operate at pressures between 14 and 16 mmHg).
Surgical technique
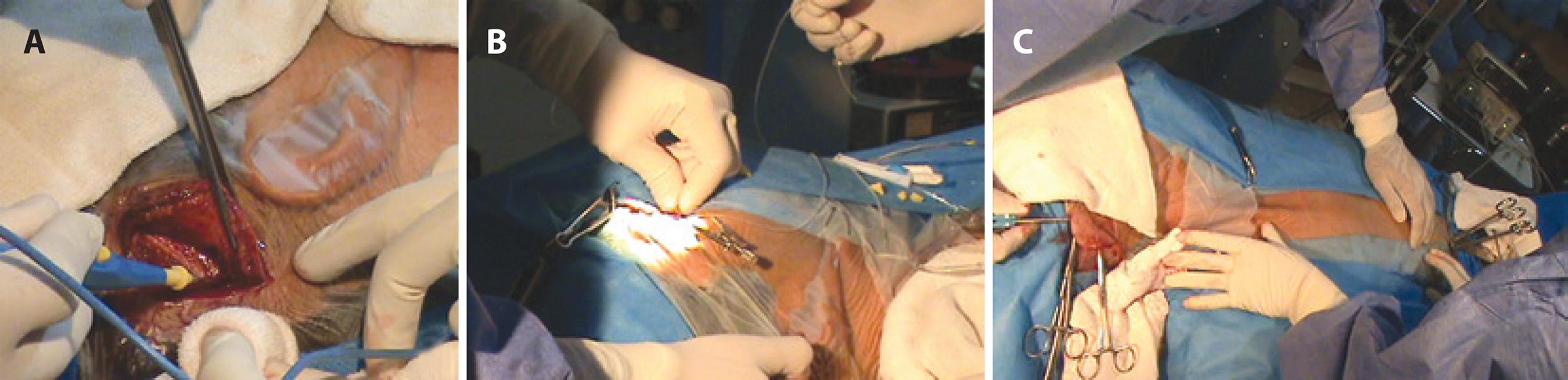
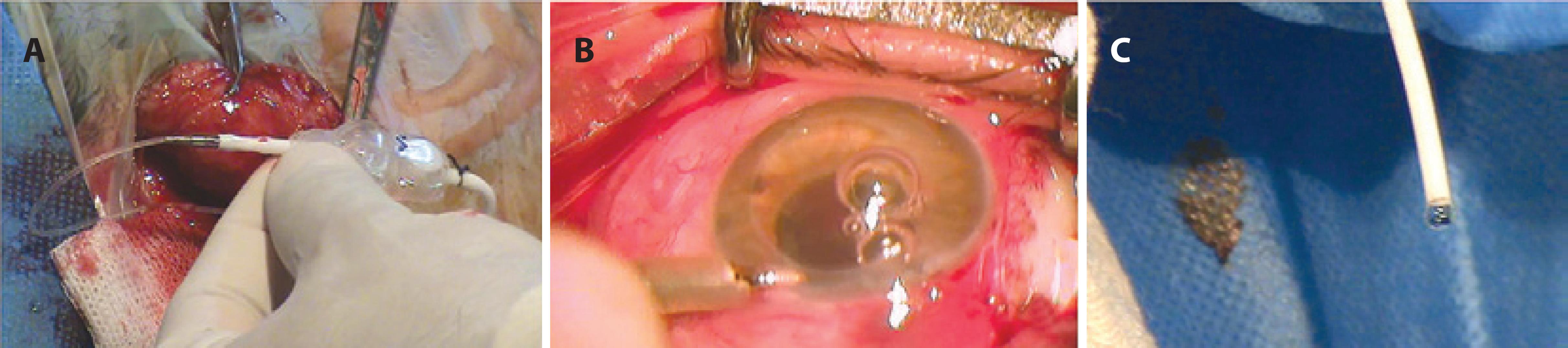
The surgical procedure begins with the formation of a fornix-based conjunctival flap. The conjunctiva is lifted and its rear face is incised, making an opening of sufficient size to allow a surgical guidewire to pass with a silicone tube mounted on it. An incision is made in the retroauricular region of the scalp (Figure 1A), and the ocular catheter mounted on the surgical guidewire is passed from the opening of the rear face of the conjunctiva to the scalp incision (Figure 1B). A tunneler is passed from the scalp incision to the periumbilical region (Figure 1C), and an opening is made in the skin and the peritoneum in this region. The hydrocephalus valve is positioned and the ocular and peritoneal catheters are assembled with the hydrocephalus valve (Figure 2A). Next, puncture of the anterior chamber of the eye is performed, and the ocular catheter is inserted. A chamber maintainer is placed (Figure 2B) and connected to a bottle of balanced saline solution to test the permeability of the system (Figure 2C). The peritoneal catheter is then introduced. All surgical wounds are closed.
RESULTS
For the first twenty days postoperatively, the IOP was constant at 15 mmHg. The anterior chamber had good depth at all follow-up examinations over this period. The keratoplasty became edematous during the first 72 hours, but this resolved with topical medication (prednisolone acetate).
To assess the precision with which these implants regulate pressure, during follow-up at week 4 the valve was adjusted to level 2.0 (to operate at pressures between 10 and 12 mmHg) and IOP immediately decreased to 11 mmHg (Figures 3 A-C). During the third postoperative month, the valve was regulated back to level 2.5. IOP increased to 15 mmHg over the following 24 hours.
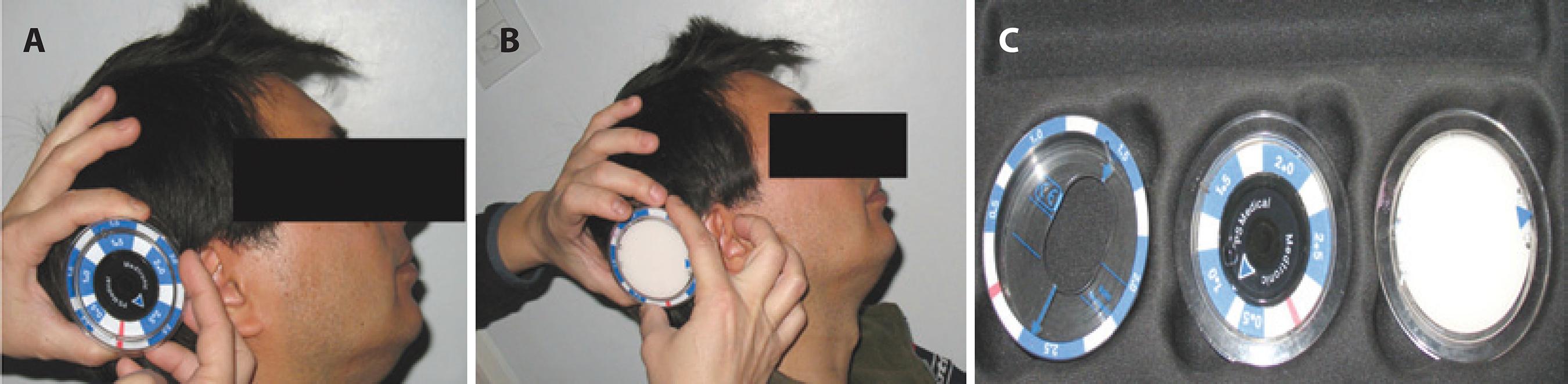
Figure 3 A) Checking the performance level of the device. B) Programming the device to level 2.0. C) Medtronic tools to adjust pressures.
On slit-lamp examination, and in the tomographic and radiological investigations in the fourth week after surgery, the catheters and valve were confirmed to be well located. (Figures 4 A-C).
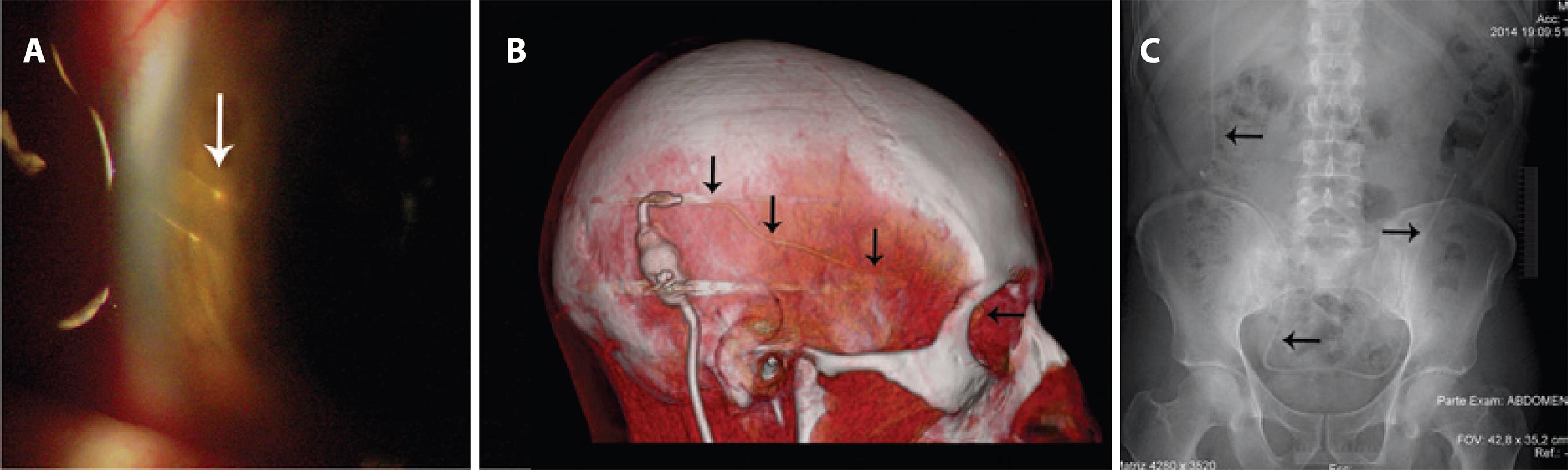
Figure 4 A) Slit-lamp examination showing the ocular catheter in position. B) Tomographic view showing the route of the ocular catheter (black arrows). C) Radiological view showing the peritoneal catheter in position (black arrows).
Although the follow-up period of this patient is not yet significant to assess the efficacy of the surgery, this is the first report in the literature of this type of shunt. Filtering aqueous humor outside the orbital cavity, thus avoiding the main reason for failure of filtering surgery, and regulating IOP in the clinic during the postoperative period without patient re-intervention, are the main focus of this research.
More studies are needed in the future to evaluate the effectiveness of this type of shunt, and to continue adapting the remarkable technological advances in implants for hydrocephalus for use in ophthalmology. A multidisciplinary approach has enabled us to successfully develop this oculo-peritoneal shunt technique.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

