INTRODUCTION
The dexamethasone implant (Ozurdex®; Allergan Inc., Irvine, CA, USA) is a novel treatment modality mainly used to treat macular edema associated with retinal vein occlusions and to treat noninfectious posterior uveitis(1). However, there are quite satisfactory data about its efficacy in multiple clinical situations associated with refractory macular edema, including diabetic macular edema, macular edema associated with uveitis or Irvine-Gass syndrome, and retinitis pigmentosa(2,3). Major concerns with intravitreal dexamethasone (IV-DEX) are increased intraocular pressure and cataract progression(1,2). Here we report an unusual case of IV-DEX-related acute retinal necrosis (ARN) in a rheumatoid arthritis patient with refractory posterior uveitis.
CASE REPORT
A 52-year-old woman presented with floaters and decreased vision in the left eye. She had a 7-year history of rheumatoid arthritis. For the year prior to presentation, she had been receiving azathioprine 150 mg daily to treat posterior uveitis in the left eye. One month prior to presentation, intravitreal dexamethasone (IV-DEX) implantation was performed for resistant macular edema in the left eye.
On examination, visual acuity was 20/20 in the right eye and 20/40 in the left eye. There were no pathological findings in the right eye. Slit-lamp examination of the left eye revealed 3+ anterior chamber cells. Fundus examination revealed vitreous haze and debris, disc edema, vessel attenuation, scattered retinal hemorrhages, exudation, and peripheral confluent areas of retinal whitening. The implant was easily observed in the inferior vitreous (Figure 1). Fluorescein angiography demonstrated staining of the optic disc and vessels, and clear-cut zones of retinal ischemia (Figure 2). Immune suppression caused by the azathioprine treatment was ruled out by a normal white cell count.
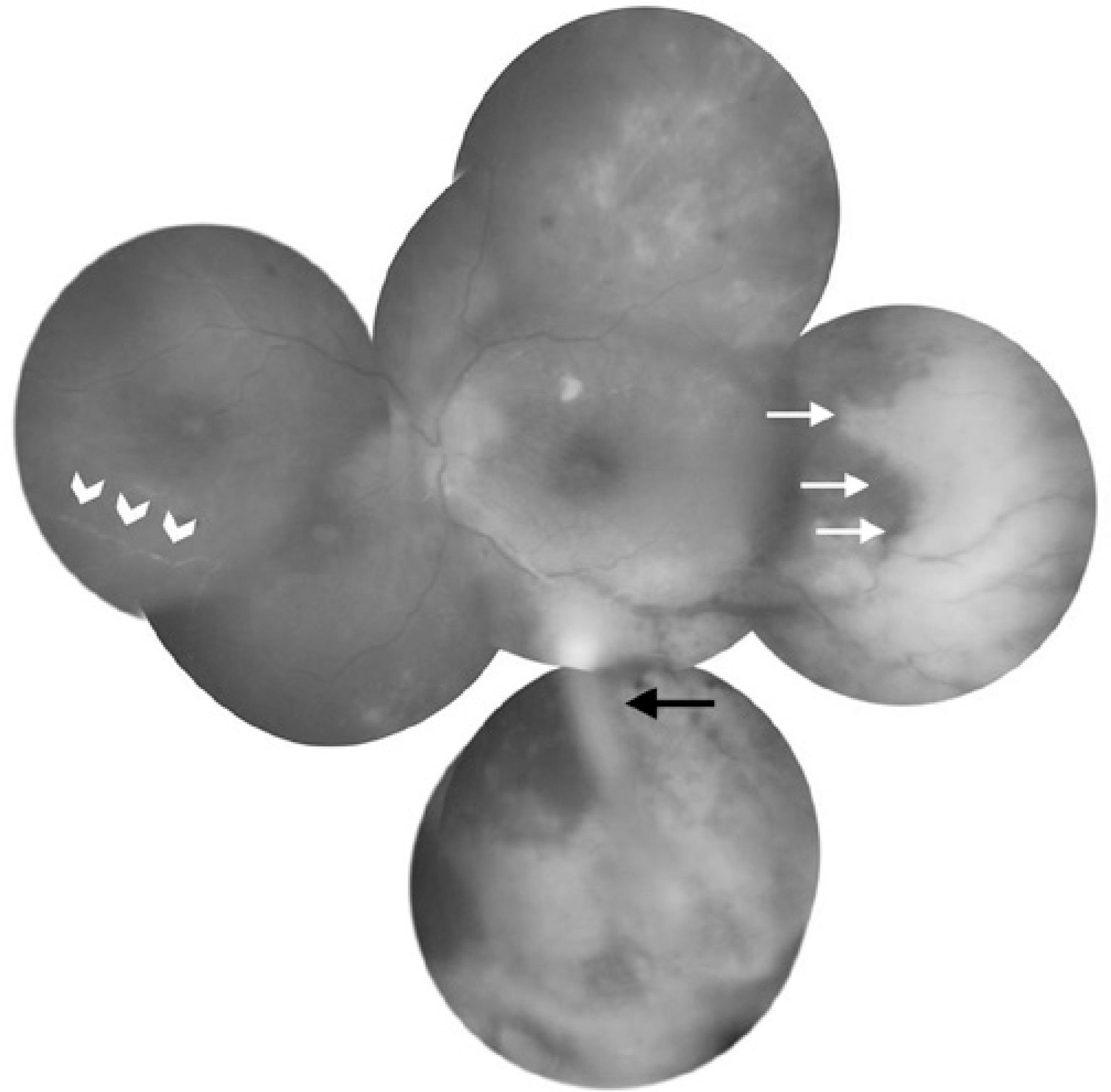
Figure 1 Fundus photography of the left eye at presentation showing mild optic disc edema, macular striations, occlusive vasculitis (white arrow heads), scattered retinal hemorrhages, clear cut whitening of peripheral retina (white arrows), and the IV-DEX implant in the inferior vitreous (black arrow).
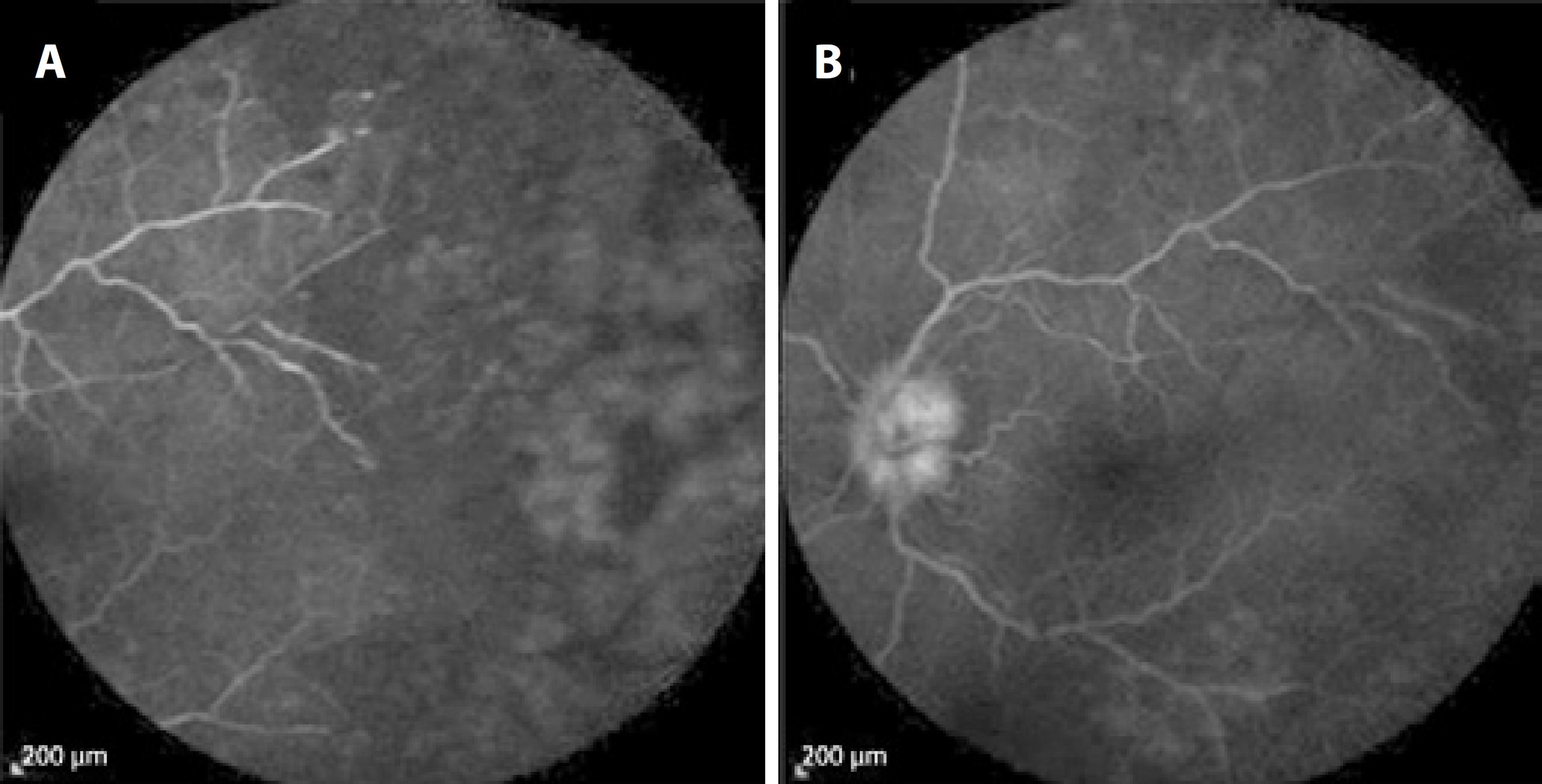
Figure 2 Fluorescein angiography images showing annular (A) retinal ischemia in the periphery and (B) optic disc leakage.
The presumed diagnosis was acute retinal necrosis. Anterior chamber paracentesis and vitreous tap samples were analyzed using viral polymerase chain reaction (PCR). Varicella zoster virus (VZV) DNA was identified, but no herpes simplex virus, cytomegalovirus, or Epstein-Barr virus DNA was detected. Systemic and laboratory investigations for syphilis, AIDS, and hepatitis were also negative, and there was no evidence of any systemic azathioprine-related adverse effect.
The patient was commenced on intravenous acyclovir (3 × 750 mg/day) treatment for 10 days to manage ARN, in addition to topical prednisolone acetate 1% drops every hour and cyclopentolate 1% drops thrice a day. The patient was followed as inpatient. However, this treatment regimen did not resolve the areas of retinal whitening or the anterior chamber inflammation. Therefore, she received 4 intravitreal ganciclovir injections a week apart, and oral valacyclovir 1 g thrice daily. One month after the first ganciclovir injection, the patient developed retinal detachment from the necrotic retinal area in the left eye, and visual acuity decreased to counting fingers at 1 m. She underwent repair of the retinal detachment with vitrectomy, laser retinopexy, and silicone oil. She was discharged after surgery and maintained on oral valacyclovir therapy. Four months after surgery, her visual acuity was 20/60 in the left eye (Figure 3). The right eye remains unaffected.
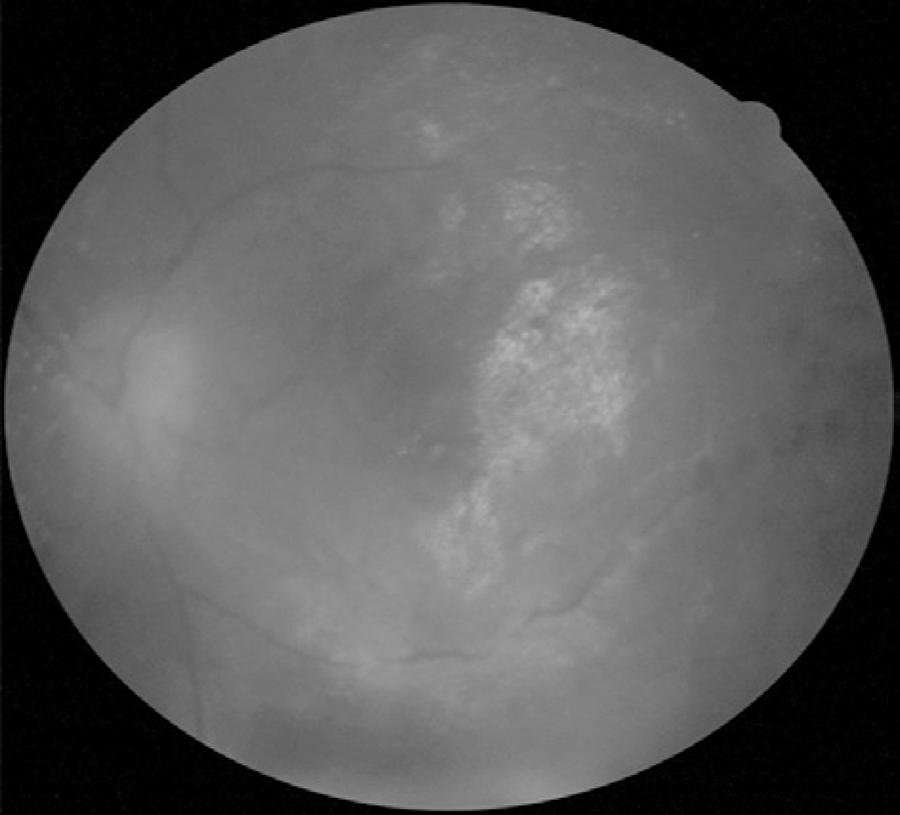
Figure 3 Fundus photography of the left eye 4 months after surgery showing attached retina under silicone oil tamponade, mild obscuration of disc edges, and some retinal scarring.
The clinical presentation and laboratory workup of the presented case lead to a diagnosis of ARN. To the best of our knowledge, there is no previous report of ARN after an IV-DEX implantation (Pubmed and Medline search). However, ARN has been described following intravitreal injections of triamcinolone(4,5). Recently, Ramaiya et al. reported a case of ARN 1 year after fluocinolone acetonide (Retisert®; Bausch and Lomb, Irvine, CA, USA) implantation in a young patient with intractable uveitis(6). Prior to implantation, the authors had tried different immunotherapeutic agents, including azathioprine with high dose oral steroid, to control the inflammation. Shah et al. retrospectively analyzed viral retinitis after triamcinolone injections and found increased number of injections or long duration of drug activity as a risk factor(5). Systemic immunosuppression is another risk factor, and its incidence was found to be 2 times higher in the immunocompromised subset than in the rest of the subjects(7). The current patient was on azathioprine for rheumatoid arthritis, and despite the normal white cell count, the combined effect of local and systemic immunosuppression could have played a role in the development of ARN. However, further studies are needed to confirm this possibility.
In the literature, early vitrectomy with laser demarcation is recommended for severe ARN(8,9). In this case, we initiated medical treatment because the macula was unaffected and visual acuity was good at presentation. After progression to retinal detachment, vitrectomy with laser retinopexy and silicone oil tamponade limited the disease progression and provided a favorable visual outcome in the short term. However, long-term follow up is needed to determine whether this unusual gain in vision is sustained.
In conclusion, physicians practicing IV-DEX implantations should be aware of this unusual but devastating complication. Extra caution and frequent follow-up is required in immunocompromised patients receiving IV-DEX implantation.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

