INTRODUCTION
Bullous keratopathy (BK) is a disorder of the corneal endothelium in which the cell density is not sufficient to regulate corneal hydration and maintain corneal clarity(1). Clinically, BK presents with stromal edema and epithelial blisters, leading to decreased visual acuity and pain(2,3). The main causes of BK are related to advanced age; trauma; absolute glaucoma; primary corneal endotheliopathies, such as Fuchs’ endothelial dystrophy; and endothelial cell loss following surgical interventions, such as cataract surgery with or without intraocular lens implantation (anterior or posterior chamber pseudophakic implants), vitreoretinal surgery (particularly when silicone oil is used), and penetrating keratoplasty (PK) following graft failure or immune rejection(1,3-7).
Corneal graft is the definitive treatment for BK, restoring vision (when possible) and providing pain relief. Other treatments can be used for patients with no potential for vision restoration or while patients are waiting for a corneal graft. Such treatments include the use of topical 5% NaCl hypertonic solution, non-steroidal anti-inflammatory agents, antiglaucomatous medications, therapeutic contact lenses, conjunctival flaps, electrocauterization, annular keratotomy, phototherapeutic keratectomy, anterior stromal puncture (ASP), and amniotic membrane transplantation (AMT)(3,8-12).
In order to compare the effectiveness of ASP and AMT for the relief of pain in patients with advanced BK(3), central corneal thickness (CCT) was considered to be an important variable, although it exceeded the upper limit for ultrasound pachymetry. Ultrasound biomicroscopy (UBM) is considered to be an alternative method for measuring CCT because it is an imaging method that provides cross-sections of the intact anterior segment of the eye at microscopic resolution. UBM has been used as an aid in diagnoses of ocular disease, as predicted by Pavlin et al. in 1991, when the method was first described(13-16) and provides highly detailed images of the cornea, allowing the characterization of structural changes of the corneal layers.
In the present study, we assessed quantitative and qualitative features of eyes with advanced BK using UBM, and its association with pain intensity before and after patients underwent ASP or AMT.
METHODS
The present descriptive comparative study was conducted over 18 months and was part of a randomized prospective study comparing ASP and AMT in the management of pain in patients with symptomatic BK. The inclusion criteria were chronic pain related to BK in patients on the waiting list for a corneal transplant or in patients whose affected eye had no or minimal visual potential and no indication for corneal transplant. Written consent was obtained from all enrolled patients. Exclusion criteria were age <18 years, presence of concurrent infection, increased eye pressure, and absence of pain.
A detailed history was obtained from each patient, including the administration of a questionnaire for assessing pain. Patients were asked to score the maximum intensity of pain experienced in the preceding one-month period on a scale of 1 to 10 (1=minimal pain and 10=unbearable pain)(3,8). Clinical ophthalmologic examinations were conducted, including best corrected visual acuity, slit-lamp examination and photographic documentation with and without fluorescein staining, and Goldman tonometry(3). Quantitative (CCT) and qualitative aspects of the cornea with BK were evaluated using UBM.
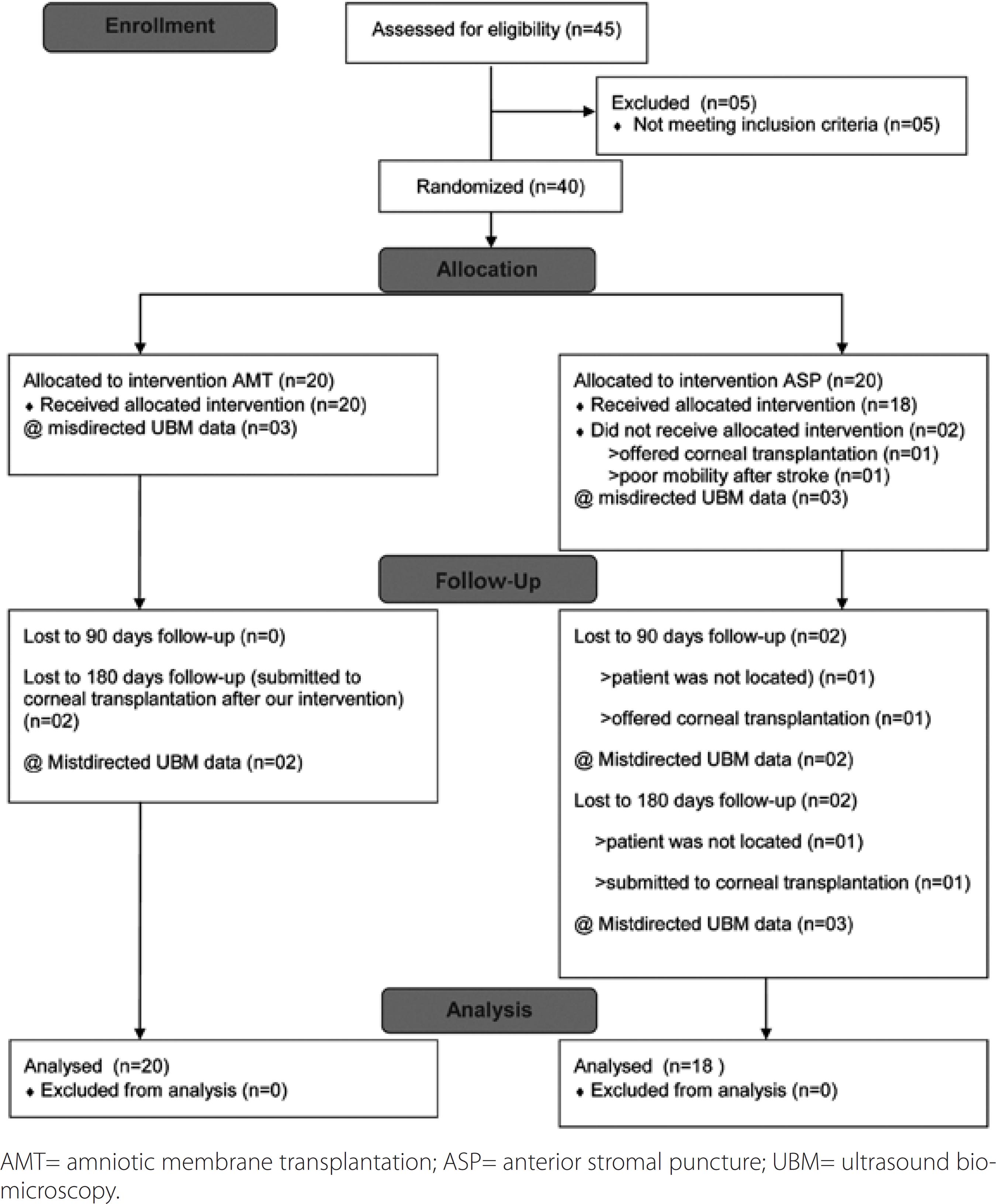
The same ophthalmologist (FSP) implemented randomization (using a computer-generated list of random numbers) as follows: 45 patients were considered, of whom 40 patients (40 eyes) were enrolled in the present study. Five patients were excluded: three of whom had previously undergone ASP, one had presented with herpes virus corneal infection, and one had raised intraocular pressure. At the outset, there were 20 patients in each group (AMT and ASP). After randomization, two patients in the ASP group were excluded from the analysis, as described in figure 1.
The causes of BK were endothelial lesion related to previous cataract surgery in 17 (85%) patients in the AMT group, and in 14 (77.7%) patients in the ASP group (p=0.102). Of the 17 patients enrolled in the AMT group, nine had additional surgical procedures: trabeculectomy (three patients; one underwent reoperation), intraocular lens removal (three patients, two with associated vitrectomy), intraocular lens fixation (one patient), glaucoma valve implant associated with PK (one patient) and vitreoretinal surgery (one patient). Moreover, of the 14 patients in the ASP group, three underwent additional procedures: trabeculectomy (one patient underwent reoperation) and intraocular lens removal (two patients)(3,17). Demographic characteristics and other clinical features are presented in table 1.
Table 1 Demographics and clinical features of patients with symptomatic bullous keratopathy divided in the two groups: anterior stromal puncture (ASP) and amniotic membrane transplantation (AMT)
| Group | |||
|---|---|---|---|
| Variable | AMT (n=20) | ASP (n =18) | p |
| Age (years) | |||
| Mean (SD) | 64.1 (15.1) | 66.2 (13.9) | 0.656 |
| Range | 23-81 | 42-85 | |
| Gender n (%) | |||
| Male | 10 (50%) | 4 (22.2%) | 0.076 |
| Female | 10 (50%) | 14 (77.8%) | |
| Affected eye | |||
| Right | 11 (55.0%) | 9 (50%) | 0.758 |
| Left | 9 (45.0%) | 9 (50%) | |
| Disease duration (years) | |||
| Mean (SD) | 2.2 (1.5) | 2.3 (2.4) | 0.624 |
| Median | 2.0 | 1.8 | |
| Range | 0.17 - 5 | 0.25 - 8 | |
| Etiology | |||
| Unknown | 0 ( 0.0%) | 3 (16.7%) | |
| Cataract surgery | 17 (85.0%) | 14 (77.7%) | 0.102 |
| Vitreorretinal surgery | 1 ( 5.0%) | 0 ( 0.0%) | |
| Fuchs' endothelial dystrophy | 2 (10.0%) | 1 ( 5.6%) | |
AMT= amniotic membrane transplantation; ASP= anterior stromal puncture; statistical tests used were student's t test or the Mann-Whitney test for independent samples, and χ2= test or Fisher's exact test.
Both ASP and AMT procedures were performed by the same ophthalmologist (FSP) as previously described by Paris et al.(3) Patients were followed up on days 1, 14, 30, 90, and 180 postoperatively.
UBM was performed preoperatively and at 90 and 180 days’ follow-up. At the time of the study, the available equipment was a Humphrey UBM Model 840, 50-MHz transducer, and the examination was conducted under topical anesthesia with the aid of an immersion cup filled with 2% methylcellulose solution and 0.9% saline solution. To evaluate the central cornea, an axial scan was utilized, with 60 dB gain and 5.0 mm width x 5.0 mm depth in a vertical scan of the central cornea. If the patient was wearing a therapeutic contact lens, it was maintained during the UBM examination. Images were acquired and posteriorly analyzed, providing A-mode measurements of thickness (quantitative analysis) and B-mode features (reflectivity and anatomy; qualitative analysis). Parameters evaluated were the presence of epithelial bullae, epithelial thickening, stromal thickening, stromal reflectivity, and Descemet’s membrane regularity. In the AMT group, the amniotic membrane thickness was included in the CCT measurement. In stored images for which posterior editing was not possible, a National Institute of Health (NIH)-approved software for measurements (ImageJ64) that allows users to scale according to a given reference in the image and, subsequently, to measure the parameter of interest. ImageJ is a public-domain, Java-based imag-processing program developed at NIH. The total thickness of tissue was considered in the measurements. If there was fluid in the interface, this fluid was not included in the measurement.
Statistical analysis
Data were presented as mean, standard deviation (SD), median, minimum and maximum values, or absolute and relative frequencies (%), as appropriate. Patient characteristics and baseline variables were compared with respect to the quantitative variables using Student’s t test or the Mann-Whitney test for independent samples, and with respect to categorical variables using the χ2 test or Fisher’s exact test. Spearman’s correlation coefficient was used to measure the correlation of CCT with pain intensity preoperatively and at 90 and 180 days postoperatively(18).
Nonparametric analysis of ordered categorical data in study designs with longitudinal observation, as described by Brunner and Langer, was performed to compare CCT, epithelial and stromal thickness (ST) distributions in both groups, and to compare preoperative and at 180-day follow-up within each group(19). When differences were asssessed in ST analysis, p values were corrected by Bonferroni inequalities(20). Non-parametric analysis was required because assumptions for using a model of analysis of variance for repeated measures were not satisfied(20). p<0.05 (α=5%) was considered to be statistically significant.
RESULTS
In the 180-day follow-up, CCT and epithelial thickness (ET) increased (p<0.001) in both groups. The results for the AMT group were CCT mean value, 899.4 µm preoperatively and 1122.5 µm postoperatively; ET mean value, 156.4 µm preoperatively and 247.8 µm postoperatively. The results for the ASP group were CCT mean value, 756.7 µm preoperatively and 914.8 µm postoperatively; ET mean value, 102.1 µm preoperatively and 245.2 µm postoperatively. ST measurements increased at the 180-day follow-up in the AMT group [ST mean value, 742.9 µm preoperatively and 826.3 µm postoperatively (p=0.005)], while the ASP group did not exhibit differences in ST following the intervention [ST mean value, 654.6 µm preoperatively and 681.5 µm postoperatively (p>0.999)]. Details are presented in table 2 and figure 2.
Table 2 Descriptive statistical values for corneal central thickness, epithelial thickness and stromal thickness in AMT- and ASP-treated symptomatic eyes with bullous keratopathy preoperatively and at 90 and 180 days follow-up
| Central corneal thickness (mμ) | ||||||||
| Preoperative | AMT | 17 | 899.4 | 290.9 | 608 | 809.0 | 1603 | 330.0 |
| ASP | 15 | 756.7 | 294.6 | 463 | 718.0 | 1725 | 226.0 | |
| Total | 32 | 832.5 | 296.8 | 463 | 778.0 | 1725 | 254.5 | |
| 90 days | AMT | 20 | 1075.3 | 314.6 | 723 | 950.5 | 1750 | 457.3 |
| ASP | 14 | 849.6 | 158.8 | 561 | 839.5 | 1100 | 208.3 | |
| Total | 34 | 982.3 | 282.2 | 561 | 923.5 | 1750 | 292.8 | |
| 180 days | AMT | 16 | 1122.5 | 323.9 | 741 | 969.5 | 1720 | 583.5 |
| ASP | 13 | 914.8 | 274.9 | 573 | 862.0 | 1476 | 329.5 | |
| Total | 29 | 1029.4 | 315.7 | 573 | 943.0 | 1720 | 443.5 | |
| Epithelial thickness (μm) | ||||||||
| Preoperative | AMT | 17 | 156.4 | 72.0 | 69 | 130.0 | 296 | 124.0 |
| ASP | 15 | 102.1 | 29.2 | 52 | 105.0 | 168 | 43.0 | |
| Total | 32 | 130.9 | 61.8 | 52 | 114.5 | 296 | 43.3 | |
| 90 days | AMT | 20 | 195.0 | 108.5 | 52 | 179.5 | 440 | 129.8 |
| ASP | 14 | 212.9 | 120.3 | 84 | 185.5 | 568 | 73.5 | |
| Total | 34 | 202.4 | 112.0 | 52 | 185.5 | 568 | 107.8 | |
| 180 days | AMT | 16 | 247.8 | 111.5 | 92 | 243.5 | 425 | 226.3 |
| ASP | 13 | 245.2 | 157.6 | 76 | 162.0 | 572 | 280.5 | |
| Total | 29 | 246.6 | 131.6 | 76 | 243.0 | 572 | 245.5 | |
| Stromal thickness (μm) | ||||||||
| Preoperative | AMT | 17 | 742.9 | 276.0 | 494 | 647.0 | 1470 | 363.5 |
| ASP | 15 | 654.6 | 297.9 | 364 | 600.0 | 1610 | 241.0 | |
| Total | 32 | 701.5 | 285.3 | 364 | 619.5 | 1610 | 211.5 | |
| 90 days | AMT | 20 | 828.9 | 269.9 | 538 | 692.0 | 1418 | 410.3 |
| ASP | 14 | 636.6 | 119.3 | 370 | 630.5 | 880 | 128.0 | |
| Total | 34 | 749.7 | 238.2 | 370 | 671.5 | 1418 | 157.3 | |
| 180 days | AMT | 16 | 826.3 | 306.0 | 507 | 703.0 | 1410 | 507.5 |
| ASP | 13 | 681.5 | 250.9 | 492 | 556.0 | 1343 | 257.0 | |
| Total | 29 | 761.4 | 287.2 | 492 | 637.0 | 1410 | 318.0 | |
SD= standard deviation; AMT= amniotic membrane transplantation, ASP= anterior stromal puncture; IQ= interquartile interval. Nonparametric analysis of ordered categorical data in designs with longitudinal observations was performed.
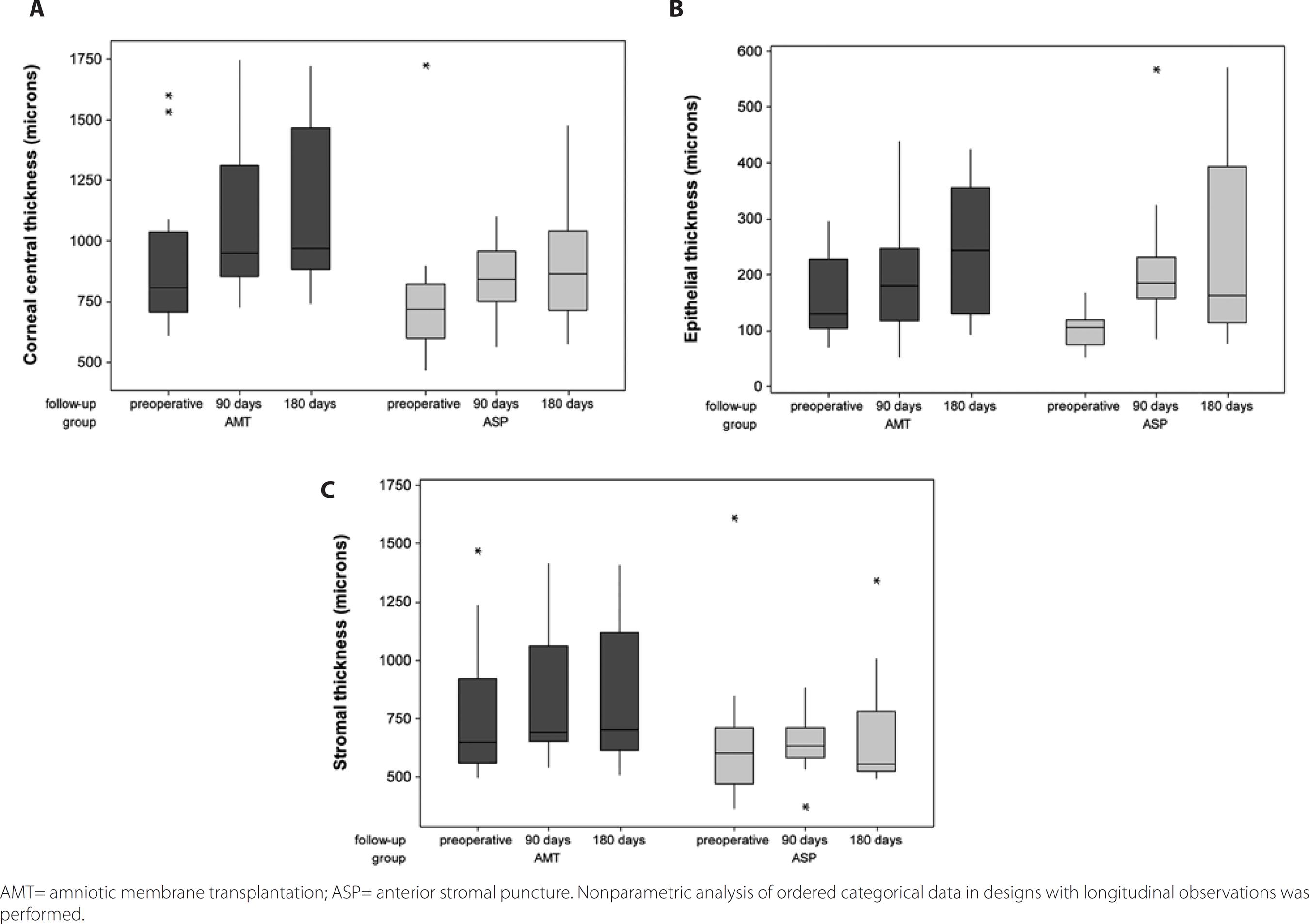
Figure 2 Box-plots of corneal central thickness (A), epithelial thickness (B), and stromal thickness (C) in AMT and ASP-treated symptomatic bullous keratopathy eyes, preoperatively and at 90 and 180 days follow-up.
The correlations between CCT and pain intensity in the AMT group (p=0.209 pre- and postoperatively) and the ASP group (p=0157 preoperatively and 0.426 at 180-day follow-up) were not significant, as seen in table 3.
Table 3 Spearman's correlation coefficient of central corneal thickness (CCT) and pain intensity in preoperative and at 90 and 180 days postoperatively
| Group | |||||||
|---|---|---|---|---|---|---|---|
| AMT | ASP | ||||||
| Follow-up | r | p | n | r | p | n | |
| Preoperative | 0.32 | 0.209 | 17 | -0.38 | 0.157 | 15 | |
| 90 days | -0.21 | 0.366 | 20 | 0.18 | 0.543 | 14 | |
| 180 days | 0.33 | 0.209 | 16 | 0.25 | 0.426 | 12 | |
AMT= amniotic membrane transplantation; ASP=anterior stromal puncture.The statistical test used was Spearman's correlation coefficient.
The main features observed in UBM were: epithelial and stromal edema, 32 patients (100%) preoperatively and 29 (100%) in the 180-day follow-up in both the AMT and ASP groups; Descemet’s membrane folds, four patients (23.5%) preoperatively and four patients (25.0%) at the 180-day follow-up in the AMT group, and three patients (20.0%) preoperatively and two patients (15.4%) at the 180-day follow-up in the ASP group; epithelial bullae, five patients (29.4%) preoperatively and five patients (31.3%) at the 180-day follow-up in the AMT group, and four patients (26.7%) preoperatively and two patients [15.4%] at the 180-day follow-up in the ASP group); and presence of fluid at the interface in three (17.6%) patients in the AMT group. See figure 3.
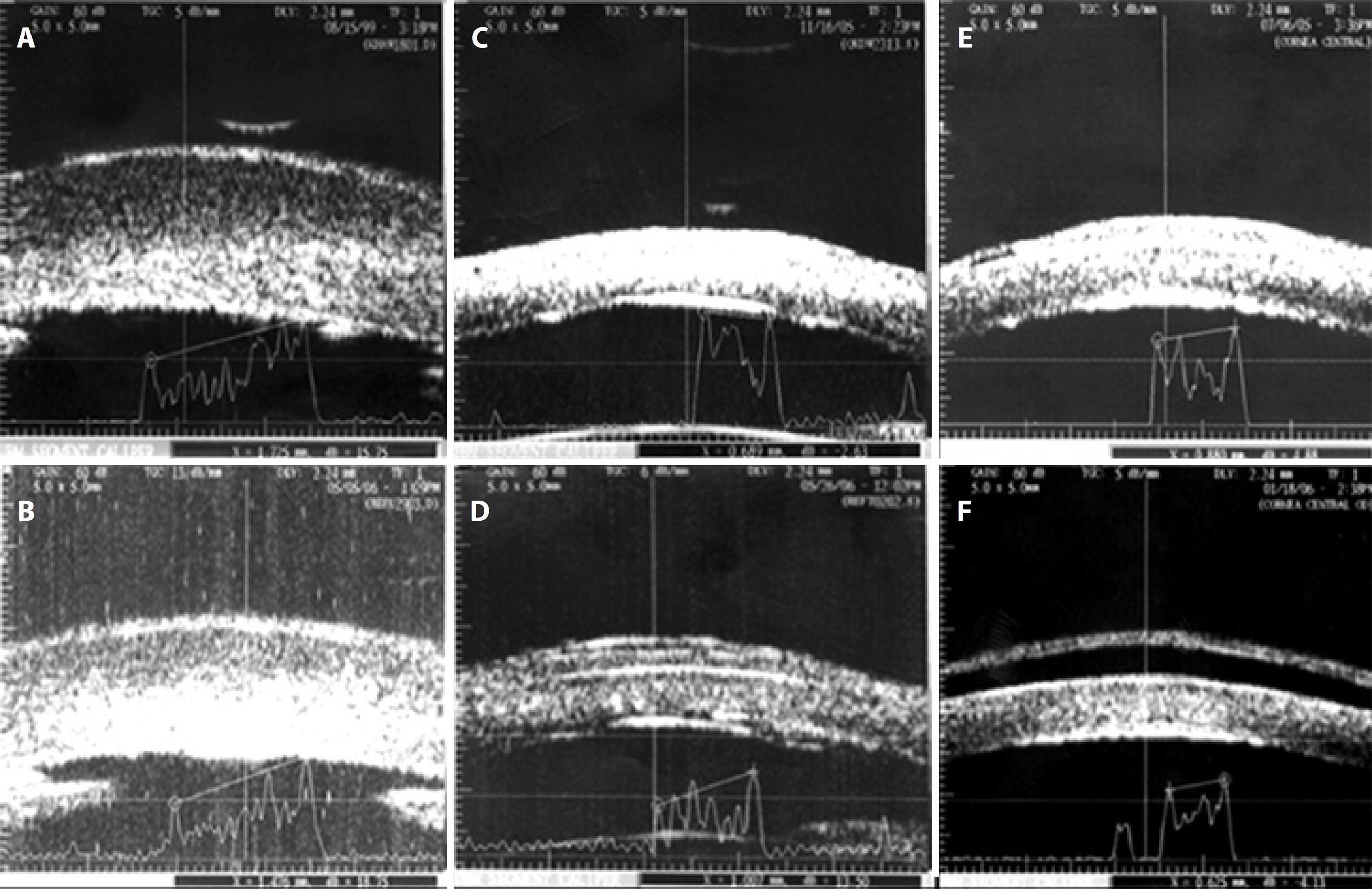
Figure 3 Ultrasound biomicroscopy in bullous keratopathy. Preoperative measures (3A, 3C, and 3E) demonstrating stromal hyper-reflectivity and thickening, epithelial thickening, and posterior surface irregularity (Descemet’s membrane folds). Total corneal thickness was found to be 1,725 (3A), 689 (3C), and 880 μm (3E). Examples of each group of treatment: 3B. Anterior stromal puncture with reduction in thickness (1,476 μm); 3D. Amniotic membrane transplantation (membrane positioned at the corneal surface, interface detected); 3F. Amniotic membrane transplantation with a complication: presence of fluid in the interface between the membrane and corneal surface.
DISCUSSION
Global trends show that after the advent of phacoemulsification, BK has become the leading indication of corneal and endothelial transplantation, particularly in developed countries(7,17,21-26). Because patients with BK can experience severe pain, many clinical and surgical interventions intending to improve the quality of life for these patients can be attempted while they are waiting for a donor cornea or when visual potential is poor and transplant is contraindicated. Previous studies suggested that ASP and AMT are efficient treatments for pain relief in patients with advanced BK(3,8-12). Frequently, UBM is important for planning the surgical approach when there is potential for restoring vision in eyes with advanced BK and corneal opacity due to severe edema. Therefore, we believe that the increasing number of aphakic and pseudophakic BK associated with the use of palliative treatments, such as AMT and ASP, will be a common finding on UBM examination.
The definitive treatment to control edema with enlarged corneal thickness in BK is a penetrating or endothelial corneal graft. It is not surprising that, at the 180-day follow-up of the present study, CCT and ET showed significantly (p<0.001) increased values in both the AMT and ASP groups because endothelial cell count was still reduced after the palliative treatments were proposed. In addition, the physical presence of the amniotic membrane contributes to the increase in CCT and ET values in the AMT group. Subepithelial fibrosis is described after AMT and ASP(3,8,27). This can constitute a barrier to fluid movement from the continuously hydrated stroma to the epithelium and can also decrease tissue evaporation, leading to stromal enlargement. This effect could be observed in the 180-day follow-up, when ST was significantly increased in the AMT group (p=0.005). Despite this, in the 180-day follow-up, ST did not significantly change in the ASP group (p<0.999); thus, CCT increased at the expense of ET enlargement in the ASP group. Technically, an increase in ST could be a negative factor in a future corneal transplantation in the AMT group compared with the ASP group, considering that suturing a very thick receptor cornea might add technical difficulty due to the difference between donor and receptor corneal thickness.
The role of the amount of edema, here expressed by CCT, in pain intensity in patients with BK is uncertain. The presence of aberrant regeneration of stromal nerves may be a factor leading to pain in patients with BK, as demonstrated by Al-Aqaba in a histological and laser scanning confocal microscopy study. Al-Aqaba et al could not reach a conclusion regarding a correlation between corneal nerves changes and corneal thickness or the duration of corneal edema due to the limited number of cases(7). Our sample constituted of advanced cases of BK, considering that the median time of beginning of symptoms was two years in the AMT group and 1.8 years in the ASP group (p=0.624), which is consistent with the elevated values of CCT observed (mean, 832.5 µm; median, 778 µm), with a large variation (463-1725 µm) considering the total number of studied eyes in the preoperative period (n=32). The correlation between CCT and pain intensity was not significant in both groups preoperatively and at the 180-day follow-up (Table 3). New studies that correlate CCT with clinical and histological data should elucidate the influence of edema and corneal nerve changes in pain related to BK.
The main UBM characteristics in eyes with BK are epithelial and stromal edema, Descemet‘s membrane folds, and epithelial bullae. In clinical practice, these features are frequently observed when the endothelial barrier and the ionic pump functions are impaired, leading to epithelial fragility, neovascularization, and corneal opacification. Also, interface fluid [three eyes (17.6%)] is a particular finding observed postoperatively in the AMT group. When the amnion-epithelial sheet is not fully attached to the corneal stroma after AMT, aqueous humor from the edematous stroma may lead to fluid accumulation in this interface. Said et al evaluated histological changes of receptor corneas of eyes with painful BK that underwent AMT before PK and observed that cells from the corneal stroma can migrate and proliferate on the amniotic stroma only through breaks in the Bowman’s zone. In these sites of migration, the membrane was firmly attached to the corneal surface and, in regions with no breaks, hydration of the cornea and accumulation of fluid continued to occur, but without bullae formation because fluid did not appear to move beyond the transplanted re-epithelialized amnion. At slit-lamp examination, this was evidenced by the observation of a fluid cleft between the epithelialized amniotic membrane and the Bowman’s zone(28). Clinically, interface fluid does not appear to have an impact on pain intensity because none of these three patients reported pain in the 180-day follow-up.
We recognize that an anterior segment optical coherence tomography (OCT) would visualize the digital sectioning of the cornea with high resolution, superior to UBM resolution. Both methods can provide the quantitative and qualitative information needed for the study; however, in contrast to OCT, UBM enables the measurement of corneal thickness >1,000 µm, independently of the degree of opacity. At the preoperative measurement, we noted that some patients had CCT >1,000 µm. Thus, UBM represents a potential alternative to measure corneal thickness and to evaluate details of densely opaque corneas in the entire sample. OCT would provide additional corneal pachymetric map with a non-contact technology. Although UBM requires immersion and could be a factor leading to an epithelial defect in fragile epithelium such as that observed in eyes with BK, therapeutic contact lenses after UBM examination was not routine.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

