

Tomás Ortiz-Basso; Agustina Galmarini; Rodolfo Luis Vigo; Joaquín Miguel Gonzalez-Barlatay; Eduardo Jorge Premoli
DOI: 10.5935/0004-2749.20180095
ABSTRACT
Purpose: To evaluate whether any topical anti-glaucoma medications increase the risk of lacrimal drainage system obstruction or whether the presence of preservatives alone is sufficient to generate obstruction.
Methods: This nested case-control study compared a group of patients with lacrimal duct obstruction who received topical anti-glaucoma medications to a control group of patients without obstruction.
Results: The medical records of 255 patients with glaucoma who consulted the Oculoplastic Section with complaints of watery eyes were reviewed. Among these patients, 59 exhibited lacrimal drainage obstruction. Ninety-four percent of patients with lacrimal drainage obstruction used beta-blockers, and 41% used prostaglandin analogs. A logistic regression model was used to adjust for age, sex, and the use of other medications. No significant differences were observed regarding the topical anti-glaucoma medications used between groups.
Conclusion: No single topical anti-glaucoma medication demonstrated a stronger association with the development of lacrimal duct obstruction.
Keywords: Lacrimal duct obstruction; Glaucoma; Preservatives pharmaceutical; Lacrimal apparatus diseases; Ophthalmic solutions
RESUMO
Objetivo: Avaliar se algum medicamento tópico anti-glaucoma aumenta o risco de obstrução do sistema de drenagem lacrimal ou se a presença de conservantes é suficiente para gerar obstrução.
Métodos: Este estudo de caso-controle aninhado comparou um grupo de pacientes com obstrução do ducto lacrimal que receberam medicações tópicas anti-glaucoma contra um grupo controle de pacientes sem obstrução.
Resultados: Foram revistos os prontuários de 255 pacientes com glaucoma que consultaram a Seção de Oculoplástica com queixas de olhos lacrimejantes. Dentre esses pacientes, 59 apresentavam obstrução da via lacrimal de drenagem. Noventa e quatro por cento dos pacientes com obstrução usaram betabloqueadores e 41% usaram análogos de prostaglandinas. Um modelo de regressão logística foi utilizado para ajustar a idade, sexo e o uso de outros medicamentos. Não foram observadas diferenças significativas em relação às medicações tópicas anti-glaucoma usadas entre os grupos.
Conclusão: Nenhum medicamento anti-glaucoma tópico único demonstrou uma associação mais forte com o desenvolvimento de obstrução do ducto lacrimal.
Descritores: Obstrução dos ductos lacrimais; Glaucoma; Conservantes farmacêuticos; Doenças do aparelho lacrimal; Soluções oftálmicas
INTRODUCTION
Primary acquired lacrimal drainage (LD) obstruction consists of a lack of tear drainage through a system that was previously permeable(1). Several factors contribute to such obstruction, but much remains to be clarified about the pathogenesis of this disease.
LD obstruction is more frequent in older adults and middle-aged women. Some of the predisposing factors for its development are associated with chronic inflammation and fibrosis(1). These factors include conjunctivitis, nasal disease, and sinusitis(2). Other studies have also identified the use of topical anti-glaucoma medications (TAGMs) as a frequent cause of obstruction(3). This type of obstruction is due to excessive scarring, similarly as drug-induced cicatricial pseudopemphigoid(4).
It is postulated that the long-term use of topical ocular medications can induce tear film instability, conjunctival inflammation, subconjunctival fibrosis, epithelial apoptosis, and corneal surface damage. These disturbances could be generated by the presence of preservatives, and they have been observed after 3 months of TAGM treatment(5).
Although the relationship between the use of TAGMs and the development of LD obstruction is known, it is unclear whether any medications are more likely to induce obstruction.
The objective of this study was to evaluate whether any TAGM, if used for at least 3 months, resulted in a higher frequency of LD obstruction or whether preservatives alone were sufficient to induce obstruction.
METHODS
A nested case-control study was conducted in a cohort of patients with glaucoma to evaluate whether the use of any TAGM was more closely associated with the development of LD obstruction. The cohort consisted of patients older than 18 years with a diagnosis of glaucoma who were receiving treatment with a TAGM. All medications contained benzalkonium chloride as a preservative. The patients were covered by the health insurance plan of Hospital Italiano de Buenos Aires between 2005 and 2014. This prepaid medical care system serves more than 142,540 members in Buenos Aires and the urban area surrounding the city. Ninety-seven percent of the population is of European descent.
The exclusion criteria were congenital obstruction of the LD system, acute conjunctivitis, chronic cicatrizing conjunctivitis, mucous membrane pemphigoid diagnosed via biopsy, orbital radiotherapy, eyelid and/or eyelash malposition, allergic rhinoconjunctivitis, previous LD or nose surgery, and LD trauma.
The medical charts of patients were reviewed. LD obstruction was defined by tearing with a positive dye disappearance test result and lacrimal duct irrigation. All patients underwent a complete oculoplastic examination.
The control group consisted of individuals with glaucoma who received treatment with a TAGM for at least 3 months but did not complain of watery eyes or exhibit LD obstructions. Three age- and sex-matched controls were randomly selected for each case.
The study was performed according to the Declaration of Helsinki, and it was evaluated and approved by the IRB of Hospital Italiano de Buenos Aires.
A descriptive analysis was performed with quantitative variables expressed as means and standard deviations and categorical variables expressed as proportions with 95% confidence intervals (CIs). The association between numeric variables was evaluated using a t-test, and that between categorical variables was examined using the chi-squared test. A logistic regression model was used to control for confounding variables. For our study, the selected unit was the patient. P<0.05 indicated statistical significance. Stata version 13 was used for statistical analysis.
RESULTS
Among the 255 reviewed patients, 59 exhibited LD obstruction. Three pat’ients with LD obstruction were excluded because of prior histories of endonasal surgeries (n=2) and dacryocystorhinostomy (n=1), resulting in a case group of 56 patients. In addition, three patients in the control group were excluded due to lagophthalmos (n=1), mucous membrane pemphigoid (n=1), and chronic cicatrizing conjunctivitis (n=1), leaving 191 participants.
In total, 94% of patients with LD obstruction used beta-blockers, compared to 83% of controls (p=0.038). Among the cases, 41% used prostaglandin analogs, versus 58% of controls (p=0.020) (Table 1).
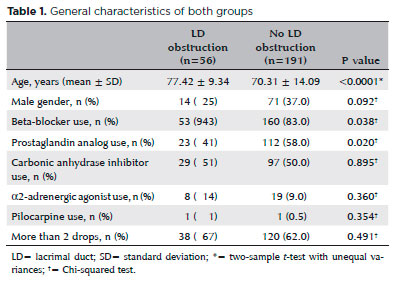
After adjusting for age, sex, and the use of other medications, no differences in the use of beta-blockers (odds ratio [OR]=2.8, 95% CI=0.75-10.89, p=0.122), prostaglandin analogs (OR=0.54, 95% CI=0.27-1.08, p=0.085), α2-adrenergic agonists (OR=1.78, 95% CI=0.67-4.7, p=0.242), or carbonic anhydrase inhibitors (OR=0.81, 95% CI=0.41-1.59, p=0.554) were observed between the two groups.
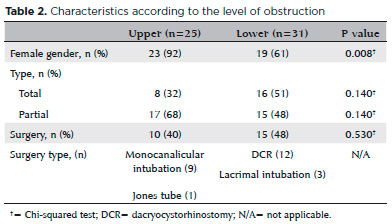
In total, 32% of upper obstructions and 51% of lower obstructions were total obstructions (Table 2). Only 45% of patients with obstruction underwent surgery.
DISCUSSION
Several studies have discussed the relationship between TAGMs and changes in the ocular surface as well as LD obstruction, but no report indicated whether any individual drug is more likely to cause LD obstruction(3). These findings suggest that no anti-glaucoma drug carries a higher-than-expected risk of LD obstruction.
The cause of LD obstruction has long been studied, and it is known to have a multifactorial etiology. Its etiology is related to advanced age, female gender, conjunctivitis, nasal diseases, and sinusitis(2,6). In addition, different researchers have proposed primary open-angle glaucoma and the use of TAGMs as predisposing factors. In particular, two prior studies found that TAGM use increased the risk of LD obstruction by 20 and 8%, respectively(7,8).
Other authors identified timolol as the main cause of obstruction. They specifically found causal relationships of the use of timolol, the number of applications, and the duration of the treatment with LD obstruction. They postulated that timolol could induce changes in the mucous of the distal end of the lacrimal apparatus, although the obstruction mechanism is unknown(9).
Conversely, some authors have argued that the use of fixed combinations and multiple drugs results in a higher degree of subclinical conjunctival inflammation. Thus, the drug combinations with higher risks of LD obstruction could include timolol + dorzolamide + pilocarpine(3,10).
While it is difficult to establish a causal direct relationship, LD obstruction and lacrimal punctum stenosis appear to be strongly associated with exposure to topic ocular medication, specifically TAGMs(3). Pathologic analysis of LD obstruction revealed swelling, vascular congestion, and edema of the nasolacrimal duct in the early stages and fibrosis with complete obstruction of the lumen in the late stages. These findings could be related to histological changes associated with TAGM use, as conjunctival metaplasia has been found, as well as decreases in goblet cell, macrophage, and lymphocyte counts at the epithelial and subepithelial levels(11).
However, the same changes were also described for the presence of preservatives, mainly benzalkonium chloride. This detergent, which disturbs cell wall permeability, has stronger pro-apoptotic and pro-inflammatory effects on the conjunctiva than preservative-free anti-glaucoma drugs(12).
This is the first study designed to identify an association of specific TAGMs with the risk of LD obstruction, and we found no evidence that any particular drug has a higher risk of LD obstruction relative to alternative treatments. Although an association between beta-blockers and LD obstruction was initially found, the association disappeared after controlling for confounding variables in logistic regression analysis. In addition, the use of multiple drugs was also not linked to a higher risk of obstruction. Therefore, LD obstruction could be caused by preservatives present in pharmaceutical preparations opposed to the effects of TAGMs themselves on the lacrimal duct.
One limitation of this study is its retrospective design. Additionally, some patients in the control group did not receive a complete oculoplastic examination, and thus, some asymptomatic cases of LD obstruction may have been misdiagnosed. Although a prospective design is necessary to confirm our hypothesis, this is a first attempt to better understand the pathogenesis of this disease.
In conclusion, although TAGMs are associated with the development of LD obstruction, no single drug appeared to have a higher risk of obstruction. Although we did not compare preservative-containing and preservative-free TAGMs, we hypothesize that preservatives have an important role in the development of obstruction. However, further studies comparing anti-glaucoma drugs with and without preservatives are required to clarify their risks of LD obstruction.
REFERENCES
1. Nemet AY. The Etiology of Epiphora: A multifactorial issue. Semin Ophthalmol. 2016;31(3):275-9.
2. Kashkouli MB, Sadeghipour A, Kaghazkanani R, Bayat A, Pakdel F, Aghai GH. Pathogenesis of primary acquired nasolacrimal duct obstruction. Orbit. 2010;29(1):11-5.
3. McNab AA. Lacrimal canalicular obstruction associated with topical ocular medication. Aust N Z J Ophthalmol. 1998;26(3):219-23.
4. Lee AJ, Mitchell P, Rochtchina E, Healey PR, Blue Mountains Eye Study. Female reproductive factors and open angle glaucoma: the Blue Mountains Eye Study. Br J Ophthalmol. 2003;87(11):1324-8.
5. Aydin Kurna S, Acikgoz S, Altun A, Ozbay N, Sengor T, Olcaysu OO. The effects of topical antiglaucoma drugs as monotherapy on the ocular surface: a prospective study. J Ophthalmol. 2014; 2014(9):460-83.
6. Nemet AY, Vinker S. Associated morbidity of nasolacrimal duct obstruction--a large community based case-control study. Graefes Arch Clin Exp Ophthalmol. 2014;252(1):125-30.
7. Kashkouli MB, Parvaresh MM, Mirzajani H, Astaraki A, Falavarjani KG, Ahadian A. Intraoperative mitomycin C use during filtration surgery and lacrimal drainage system obstruction. Am J Ophthalmol. 2009;147(3):453-7e1.
8. Ohtomo K, Ueta T, Toyama T, Nagahara M. Predisposing factors for primary acquired nasolacrimal duct obstruction. Graefes Arch Clin Exp Ophthalmol. 2013;251(7):1835-9.
9. Seider N, Miller B, Beiran I. Topical glaucoma therapy as a risk factor for nasolacrimal duct obstruction. Am J Ophthalmol. 2008; 145(1):120-3.
10. Kashkouli MB, Pakdel F, Hashemi M, Ghaempanah MJ, Rezaee R, Kaghaz-Kanani R, et al. Comparing anatomical pattern of topical anti-glaucoma medications associated lacrimal obstruction with a control group. Orbit. 2010;29(2):65-9.
11. Kashkouli MB, Rezaee R, Nilforoushan N, Salimi S, Foroutan A, Naseripour M. Topical antiglaucoma medications and lacrimal drainage system obstruction. Ophthal Plast Reconstr Surg. 2008; 24(3):172-5.
12. Pisella P-J, Debbasch C, Hamard P, Creuzot-Garcher C, Rat P, Brignole F, et al. Conjunctival proinflammatory and proapoptotic effects of latanoprost and preserved and unpreserved timolol: an ex vivo and in vitro study. Invest Ophthalmol Vis Sci. 2004;45(5): 1360-8.
Submitted for publication:
November 28, 2017.
Accepted for publication:
March 14, 2018.
Funding: No specific financial support was available for this study
Disclosure of potential conflicts of interest: None of the authors have any potential conflicts of interest to disclose
Approved by the following research ethics committee: Hospital Italiano de Buenos Aires (IRB00003580)