INTRODUCTION
More than 90 million people worldwide are affected by diabetic retinopathy (DR), causing significant vision loss(1,2), with macular edema and proliferative retinopathy as the leading causes of visual impairment(2,3). Anti-vascular endothelial growth factor (anti-VEGF) drugs are widely used in the treatment of diabetic macular edema (DME); this is supported by an extensive body of literature, demonstrating substantial improvements in visual and anatomic outcomes(4-10).
The choroid is a vascularized tissue that plays a vital role in providing metabolic support to the outer retina, including the photoreceptors and prelaminar portion of the optic nerve head(11,12). Abnormal choroidal blood volume and compromised flow may result in dysfunctioning of the photoreceptor and mortality(13). Choroidal vasculopathy, such as obstruction of the choriocapillaris, vascular degeneration, choroidal aneurysms, and neovascularization, may be involved in the pathogenesis of DR(13-15).
Enhanced depth imaging using spectral domain optical coherence tomography (SD-OCT) enables improved visualization of the choroid and assessment of choroidal thickness(16,17). Results of clinical studies have indicated that choroidal thickness may be related to DR severity, and the presence of DME is associated with a significant decrease in choroidal thickness(16). In patients with neovascular age-related macular degeneration (AMD), anti-VEGF drugs have been shown to induce significant thinning in choroidal thickness, which may lead to unknown long-term consequences or complications(18).
Currently, intravitreal anti-VEGF injections are the most common treatment for DME. Though anti-VEGF injections improved visual acuity, several studies demonstrated an association with decreased central retinal thickness(18,19). However, there is currently a lack of studies investigating the effect of anti-VEGF injections on the choroid in patients with diabetes. The purpose of the present study was to evaluate the effect of intravitreal anti-VEGF injections on choroidal thickness using SD-OCT in patients treated for DME.
METHODS
This was a retrospective chart analysis conducted at the Federal University of São Paulo (UNIFESP) and Hospital Oftalmológico de Brasília, Brazil. The present study was approved by the UNIFESP Institutional Review Boards (CEP 1.093.853) and adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from all subjects after explanation of the nature of the present study.
Subjects
Exploratory chart review of data collected from patients with type 2 diabetes previously treated for DME with intravitreal anti-VEGFs between January 2012 and June 2014. Clinically significant macular edema (CSME) was assessed by clinical examination and SD-OCT imaging. The diagnosis of CSMA was defined according to the Early Treatment Diabetic Retinopathy Study (ETDRS) protocol. A pro re nata (PRN) protocol was used to treat DME in all patients. Major exclusion criteria included the following: previous anti-VEGF or intravitreous corticosteroid treatment for DME within 6 months of the first visit; vitreomacular traction syndrome; suspected or confirmed glaucoma; proliferative diabetic retinopathy; previous retinal laser therapy; previous pars plana vitrectomy; high myopia and age-related macular degeneration; and other causes of macular edema.
Choroidal thickness measurement
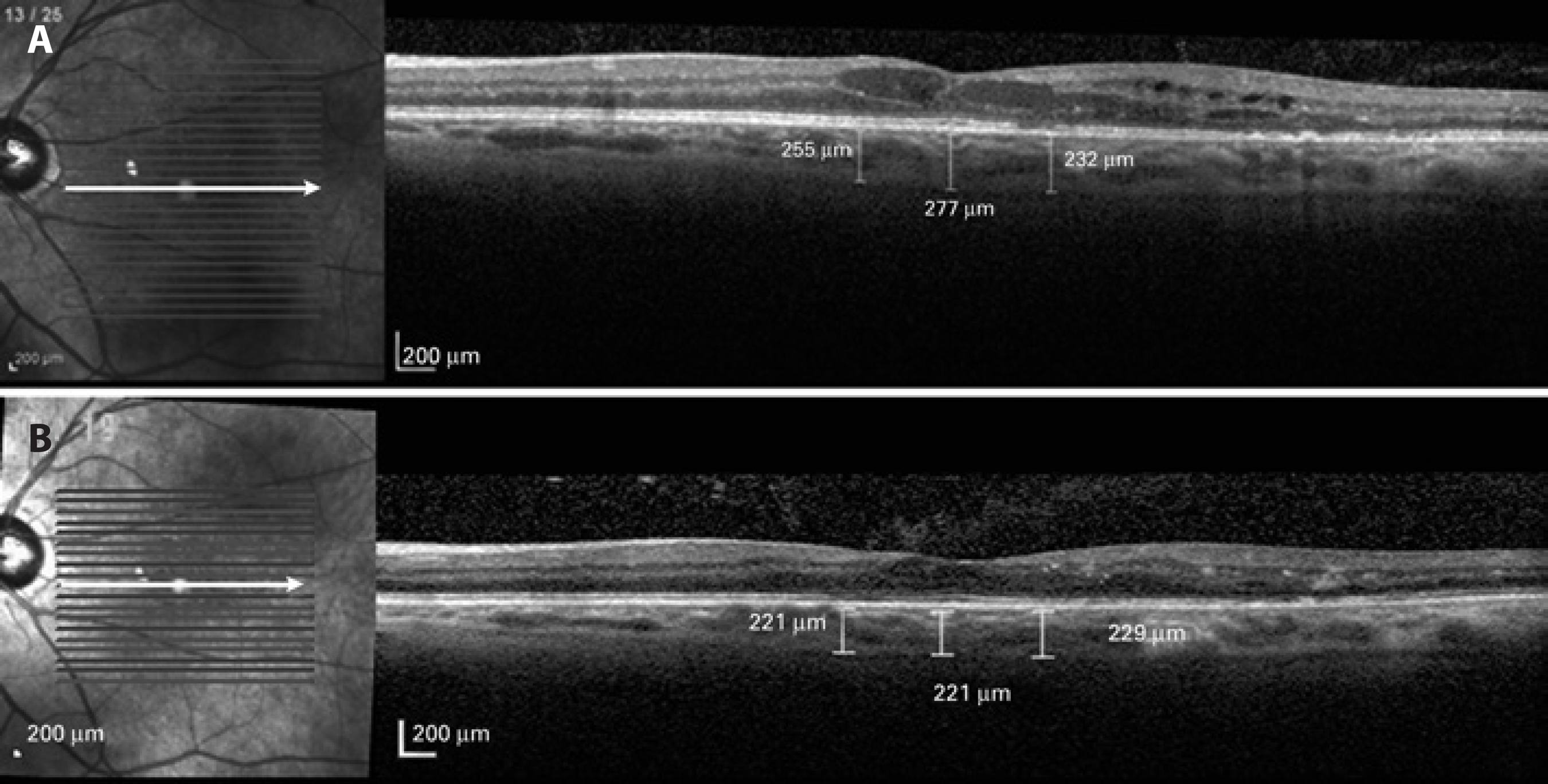
SD-OCT scans were performed using EDI SD-OCT (Spectralis OCT, Heidelberg Engineering, Heidelberg, Germany). The scan pattern used was a high-definition 1-line raster scanning of 30° consisting of 768 A-scans per frame, followed by an average of 100 frames(20). Measurements were performed before and after anti-VEGF treatment. For images to be included in the present study, the signal-to-noise ratio (SNR) had to be at least 20 dB (considered best quality) and should be taken as close to the fovea as possible (thinnest macular point), with the understanding that slight differences in positioning affect the measured thicknesses(21). Using the Spectralis linear measurement tool, two independent observers measured CT perpendicularly from the outer edge of the hyper-reflective RPE to the inner sclera at 500-mm intervals temporal and nasal to the fovea up to 1,000 mm. Measurements were performed prior to treatment and at a 6-month follow-up (Figure 1).
Statistical analysis
Data are expressed as means ± standard error of the mean. Statistical analyses were performed using a paired t-test for comparing between baseline and follow-up choroidal thickness measurements. The Pearson correlation coefficient was used to evaluate the correlation between choroidal thickness and central foveal thickness. A 95% confidence interval with a 5% level of significance was adopted; thus, P values of <0.05 were considered to be statistically significant. Analysis of variance (ANOVA) with the Bonferroni correction was used to compare groups treated with bevacizumab and ranibizumab. All statistical analyses were performed using Graph Pad Prism 5.0 software for MAC.
RESULTS
Thirty-nine eyes from 39 patients (15 female, 24 male) were included in the study analysis. The mean age was 62.43 ± 8.7 years, with a range of 44-79 years. Twenty-three eyes were treated with ranibizumab and 16 eyes were treated with bevacizumab. The average number of anti-VEGF injections administered was 2.28 ± 1.27 (range, 1-5) over the 6-month follow-up. Central retinal thickness improved in all patients after 6-month anti-VEGF treatment.
The choroid was noted to be thinnest nasally, thicker temporally, and thickest in the subfoveal region. This pattern was observed at baseline and after the 6-month follow-up, which is consistent with that reported in previous studies(22). Figure 2 shows the mean CT at each location. Choroidal thickness was significantly thinner (p=0.0327) at all measured points following anti-VEGF treatment. Mean nasal, subfoveal, and temporal choroidal thickness measurements at baseline were 234.10 ± 8.63 µm, 246.89 ± 8.94 µm, and 238.12 ± 8.20 µm, respectively, and at the 6-month follow-up were 210.46 ± 8.00 µm, 215.66 ± 8.29 µm, and 212.43 ± 8.14 µm, respectively.
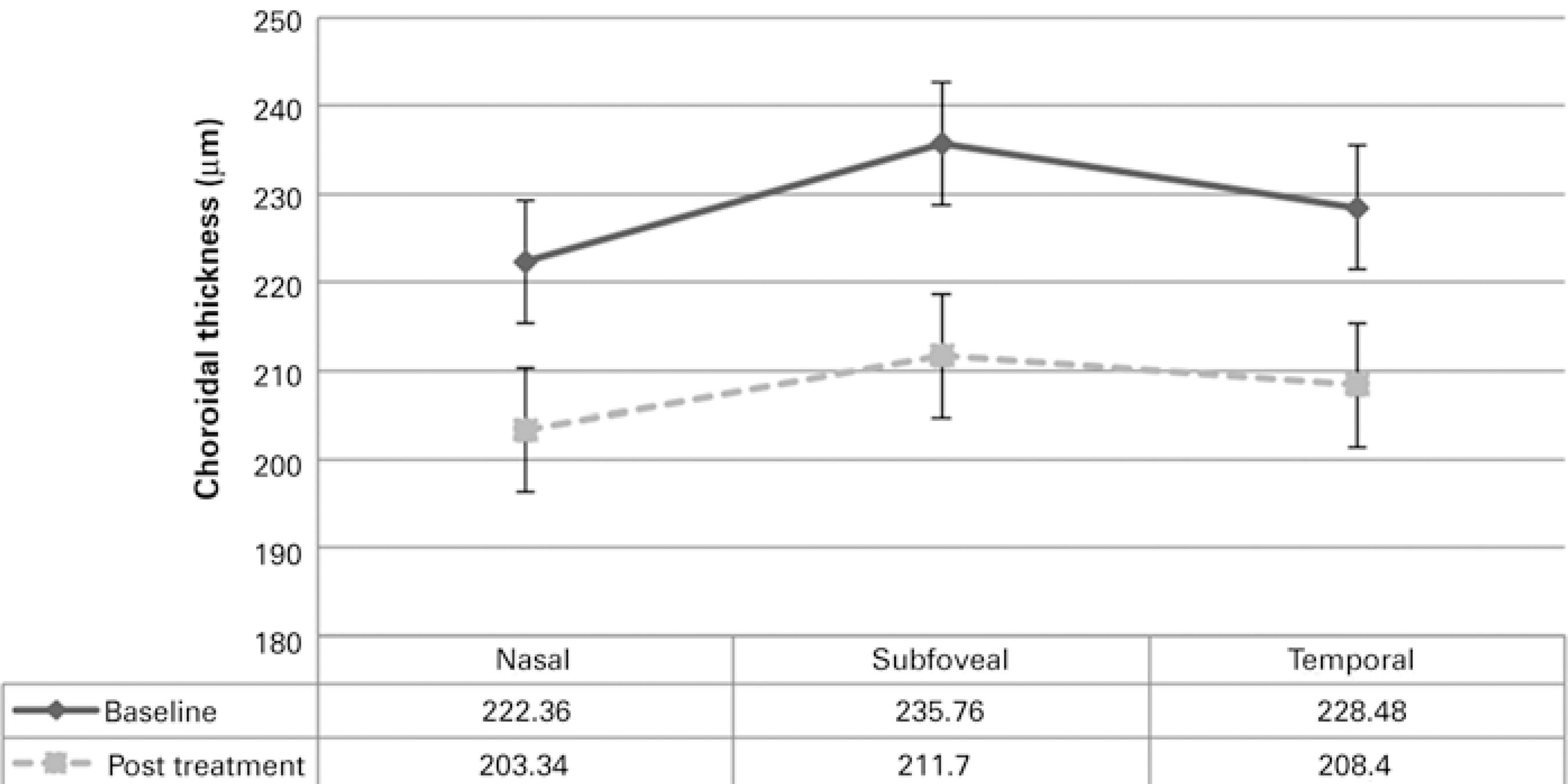
Figure 2 Mean choroidal thickness at the fovea and at 500-µm intervals nasally and temporally at baseline and at the 6-month follow-up.
In a subgroup analysis of patients who received intravitreal bevacizumab, mean nasal, subfoveal, and temporal choroidal thickness measurements at baseline were 241.25 ± 14.40 µm, 259.50 ± 13.93 µm, and 249.87 ± 13.30 µm, respectively, with significant decreases to 212.75 ± 12.70 µm, 221.12 ± 12.26 µm, and 221.12 ± 12.06 µm, respectively, at the 6-month follow-up. In the ranibizumab group, mean nasal, subfoveal, and temporal choroidal thickness measurements at baseline were 229.13 ± 10.83 µm, 238.13 ± 11.56 µm, and 229.95 ± 10.28 µm, respectively, with significant decreases to 208.86 ± 10.53 µm, 211.87 ± 11.34 µm, and 206.40 ± 11.00 µm, respectively, at the 6-month follow-up. No significant difference (p>0.05) in foveal CT was observed between eyes treated with ranibizumab (211.87 ± 11.34 µm) and those treated with bevacizumab (221.12 ± 12.26 µm). Decreases in central retinal thickness were not correlated with choroidal thinning after 6-month anti-VEGF treatment.
DISCUSSION
The choroid plays an integral role in the metabolic support of the retinal pigment epithelium (RPE), optic nerve, and outer retina; normal choroidal vasculature is essential for appropriate functioning of the retina(11,12). Compromised choroidal blood flow may result in dysfunctioning of the photoreceptor and insufficient removal of metabolites generated by RPE cells, resulting in an accumulation of waste at Bruch's membrane(13). Previous studies have reported abnormal choroidal findings in patients with diabetes such as increased blood vessel tortuosity, focal vascular dilation and narrowing, hypercellularity, vascular loops, microaneurysms, areas of non-perfusion, and sinus-like structure formation between the choroidal lobules(14,23). Choroidal thinning, increased thickness of the extracellular matrix, and decreased vessel diameter have been observed on SD-OCT imaging in the eyes of patients with diabetes(16,24,25).
Anti-VEGF treatment has demonstrated utility in improving visual and anatomical outcomes in DME(4-10). The effects of anti-VEGF therapy on choroidal thickness in various diseases, including AMD and DM, have been reported in several previous studies(18,19). Yiu et al. demonstrated that central choroidal thickness decreases following anti-VEGF therapy for DME after 6 months(19). The present study also found a statistically significant decrease in choroidal thickness among patients following anti-VEGF injections.
The results of the present study indicate that intravitreal anti-VEGF therapy may influence choroidal structure in eyes with diabetic retinopathy treated for DME. This result may be attributable to the findings that both full-length antibody (bevacizumab) and Fab fragment (ranibizumab) drugs are able to penetrate into all retinal layers, reach the choroid, and accumulate in the wall of the choroidal vessel(26-28). As VEGF functions by inducing vessel dilation and increasing ocular blood flow, which is mediated by increased nitric oxide production(29), VEGF inhibition by these drugs may cause constriction of the choroidal vessels. Choroidal thickness is already decreased in patients with diabetes, and the consequences of further thinning of the choroidal vasculature secondary to anti-VEGF administration are unknown. Altered choroidal blood flow may result in dysfunctioning of the photoreceptor and mortality, contributing to the higher anatomical and functional responses to anti-VEGF treatment in patients with thicker choroids, as demonstrated by a previous report(30).
One of the major limitations of the present study was the retrospective design. We were unable to determine which anti-VEGFs were administered, and we were unable to identify a control group. Additionally, visual acuity data were not analyzed as the major objective of the present study was to evaluate changes in choroidal thickness.
As anti-VEGF drugs are commonly used in the treatment of DME and other retinal pathologies, a full understanding of the effects of anti-VEGF drugs on the choroidal vasculature is warranted. Further prospective studies are required to completely determine the long-term effects of anti-VEGF therapy on choroidal thickness. Decreased choroidal thickness induced by anti-VEGF administration may have long-term adverse effects on choroidal and retinal functions.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

