INTRODUCTION
Aggressive posterior retinopathy of prematurity (APROP) is the most severe form of ROP characterized by apparent disproportionate plus disease compared with peripheral disease findings in Zone I or posterior Zone II(1). The diagnosis of APROP is clinically important in ensuring timely intervention as APROP can rapidly progress to tractional retinal detachment(2). Further, higher rates of unfavorable structural and refractive outcomes have been reported following laser ablation and cryoablation for the treatment of APROP(3-5).
In recent years, a number of studies have reported promising results with anti-vascular endothelial growth factor (VEGF) inhibitors, particularly bevacizumab, for the treatment of APROP(6-9). Lesser refractive errors have been demonstrated following intravitreal bevacizumab (IVB) therapy compared to laser treatment(10-13). However, there have been few studies assessing refractive and functional outcomes following IVB treatment for APROP. We therefore aimed to determine 2-year clinical outcomes following IVB monotherapy for APROP at a referral hospital for ROP patients in Turkey.
METHODS
Following institutional review board approval, we performed a retrospective review of the medical records of children who received IVB (Group I) as a single treatment modality for APROP. Further, we reviewed a group comprising infants who underwent laser treatment for APROP, designated group II. The study followed the tenets of the Declaration of Helsinki.
All cases were followed-up at Zeynep Kamil Maternity and Children's Disease Training and Research Hospital. Routine ROP screening examinations were performed at postnatal weeks 4 to 6 in all infants and follow-up examinations were performed in accordance with the relevant recommended guidelines(14). The diagnosis of APROP was made according to the “The International Classification of ROP”(1). All parents provided informed consent prior to all interventions.
Infants in group I were treated with IVB monotherapy according to the methods proposed by the Bevacizumab Eliminates the Angiogenic Threat of ROP (BEAT-ROP) study(9). All injections were performed at an office-based injection clinic. Following topical anesthesia with 0.5% proparacaine HCl and the application of 5% povidone iodine to the ocular surface, 0.625 mg (0.025 ml) bevacizumab (Altuzan, 100 mg/4 ml flacon, Roche, Turkey) was injected into the vitreous cavity using a 31-gauge needle 1 mm behind the limbus. Central retinal artery patency was confirmed immediately after each procedure. An on-site pediatrician was present during all interventions. IV B injections were performed in both eyes at the same session. Topical antibiotic drops were administered for 1 week postoperatively. A positive response to IVB therapy was defined as regression of tunica vasculosa lentis, plus disease, and neovascularization with vessels vascularizing the peripheral retina. In cases where reactivation of ROP was observed, such as the reappearance of plus disease or recurrence of proliferative components, additional IVB injections were administered. Continued examinations were performed at 1 and 2 weeks and then monthly intervals up to a mean post-menstrual age (PMA) of 79.28 ± 6.63 weeks (range, 69 weeks to 96 weeks) in order to evaluate neovascularization of the peripheral retina.
Infants in group II were treated with laser ablation according to the methods of the Early Treatment for ROP (ETROP) study(15). Following topical anesthesia with 0.5% proparacaine HCl, laser photocoagulation was performed using an 810 nm head-mounted diode laser (Iridex; Oculight SL, Mountainview, CA, U.S.A). Near-confluent laser burns were applied to the entire avascular retina. Laser ablation was also applied to areas of clinically avascular retina within the vascularized posterior retina as suggested previously(16). Repeated laser sessions were performed at 1 week after the initial laser treatment when required. Consecutive examinations were performed at weekly or monthly intervals after laser treatment up to a mean PMA of 64.73 ± 4.93 weeks (range, 57 weeks to 74 weeks) to ensure stabilization of retina.
Refractive measurements were assessed with correction to 2 years of age. Refractive errors were evaluated 45 minutes after three instillations of cyclopentolate 1% at 10-minute intervals by using a hand-held autorefractometer (Welch Allyn; Sure Sight Autorefractor, NY, USA). Three measurements were taken for each subject. In cases where inconsistencies were observed between consecutive measurements, refractive assessments were continued until at least three coherent refractive values were obtained. Refractive values were recorded in units of spherical equivalent (SE) power. Myopia was defined as a SE of ≤-0.25 diopters (D). Refractive anisometropia was defined as a difference of 1 D between SE values for each eye.
Statistical analyses
Number Cruncher Statistical System (NCSS) 2007 & Power Analysis and Sample Size (PASS) 2008 statistical software programs were used for all statistical analyses. Descriptive statistical values are presented as mean, standard deviation, frequency, minimum, and maximum. To compare quantitative and qualitative data between groups, the Mann-Whitney U test, Pearson‘s chi-square test, Yates' correction for continuity, and Fisher's exact test were used according to data distributions. Logistic regression analysis was performed to identify risk factors for refractive error. P-values <0.05 were considered statistically significant.
RESULTS
A total of 78 eyes of 40 patients were included in the present study. All patients received treatment in both eyes except 2 patients in group I who received unilateral IVB injections. No significant differences in gestational age (GA), birth weight (BW), or gender were observed between group I and group II (P=0.187 for GA; P=0.685 for BW; and P=1.000 for gender). Infant demographics are presented in table 1. Treatments were administered up to a mean PMA of 33.96 ± 1.86 and 33.93 ± 2.15 weeks in group I and group II, respectively (P=0.711). Zone 1 APROP was detected in 25 eyes (55.1%) and 18 eyes (60%), and Zone II posterior APROP was detected in 23 eyes (47.9%) and 12 eyes (40%), in group I and group II, respectively (P=0.494).
Table 1 Patient demographics
| Group I (n=25) | Group II (n=15) | P-value | |
|---|---|---|---|
| GA (weeks, mean ± SD) | | 26.40 ± 1.82 | 27.30 ± 1.82 | a0.187 |
| BW (g, mean ± SD) | 901.40 ± 304.60 | 941.00 ± 282.48 | a0.685 |
| Male/female | 11/14 | 6/9 | b1.000 |
a= Student't t test;
b= Yates' continuity correction.
Group I= Infants who received intravitreal bevacizumab monotherapy; Group II= infants who underwent laser treatment; GA= gestational age; BW= birth weight; SD= standarc deviation.
No complications related to IVB injections were observed, including iatrogenic cataract, endophthalmitis, retinal detachment, and vitreous hemorrhage. All eyes in group I demonstrated total regression of plus disease, neovascular proliferation, persistent tunica vasculosa lentis if previously present, and vitreous haze on examination at 1 week post-injection. However, three infants had recurrence of vascular engorgement with newly burgeoning proliferative tissues requiring a second treatment with IVB at a mean PMA of 39.66 ± 1.69 weeks (range, 38 weeks to 42 weeks). Anatomic outcomes were successful in all patients in group I at the end of the follow-up period.
Two cases in group II required a second round of laser therapy at 1 week after initial laser treatment consisting of laser ablation of avascular areas after retraction of flat neovascular networks. Further, two eyes in group II had stage 4A retinal detachments which remained stable without new vessel formation during the follow-up period. Progression to stage 4B or stage 5 retinal detachment was not observed in any patient.
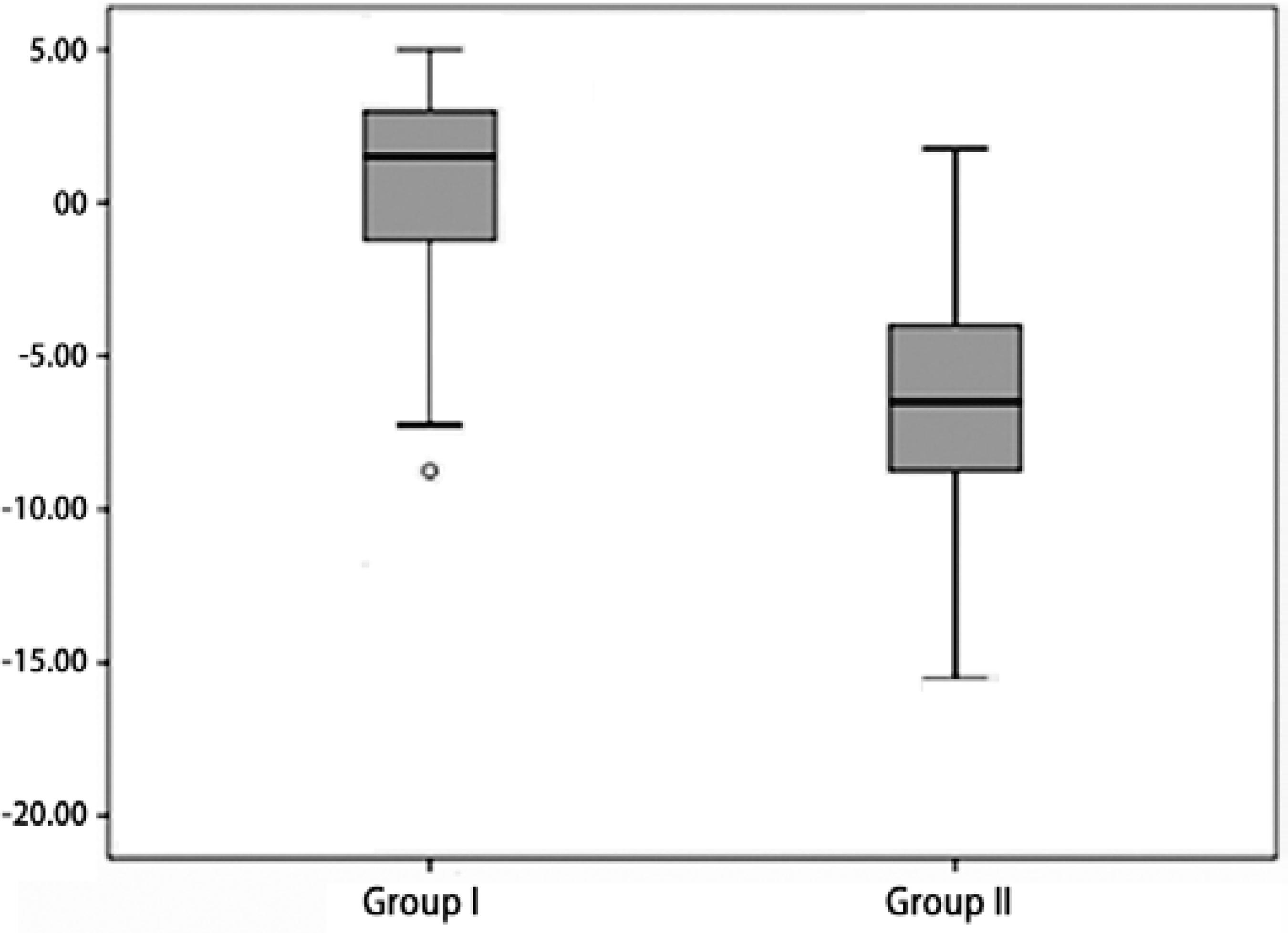
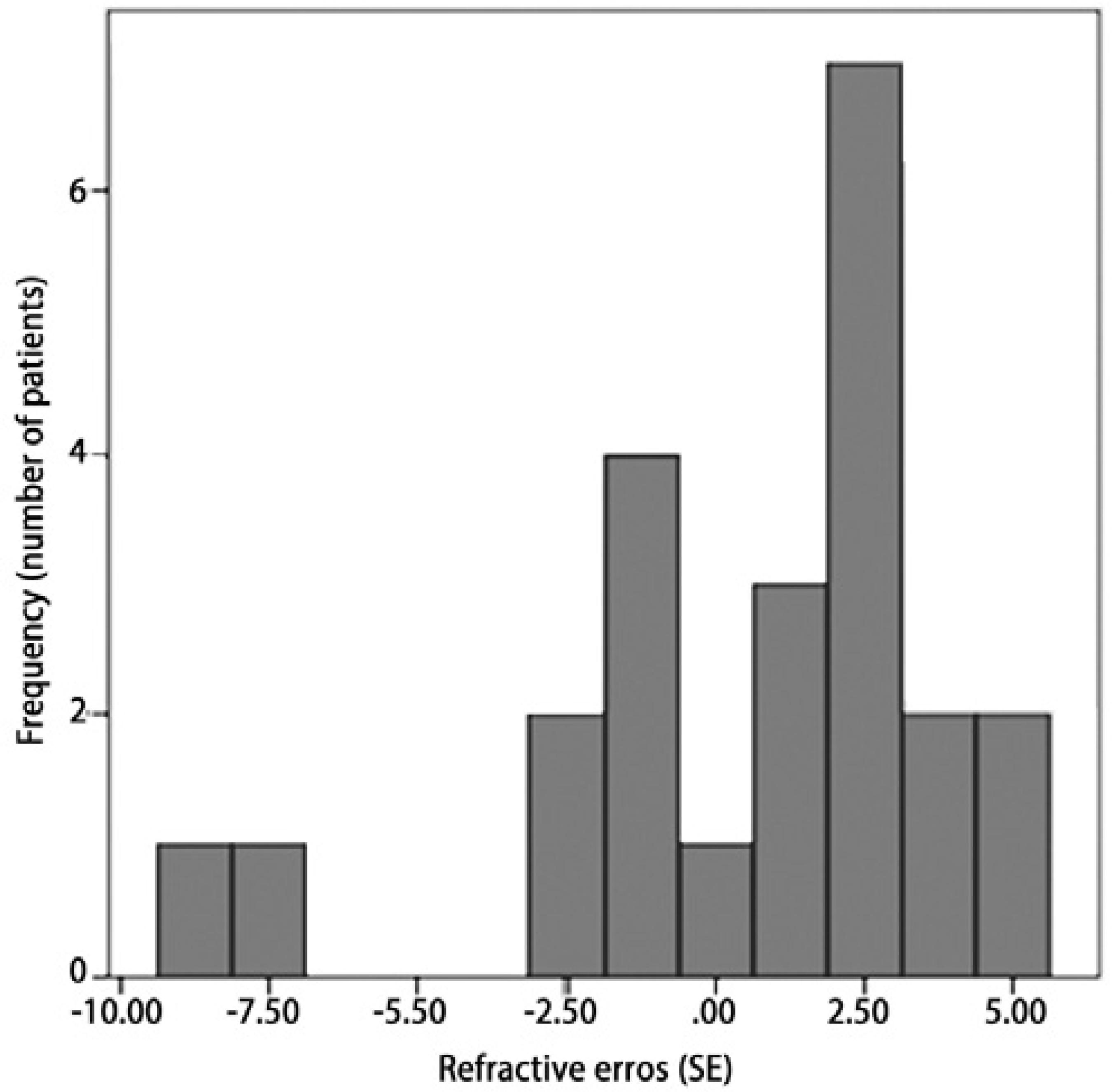
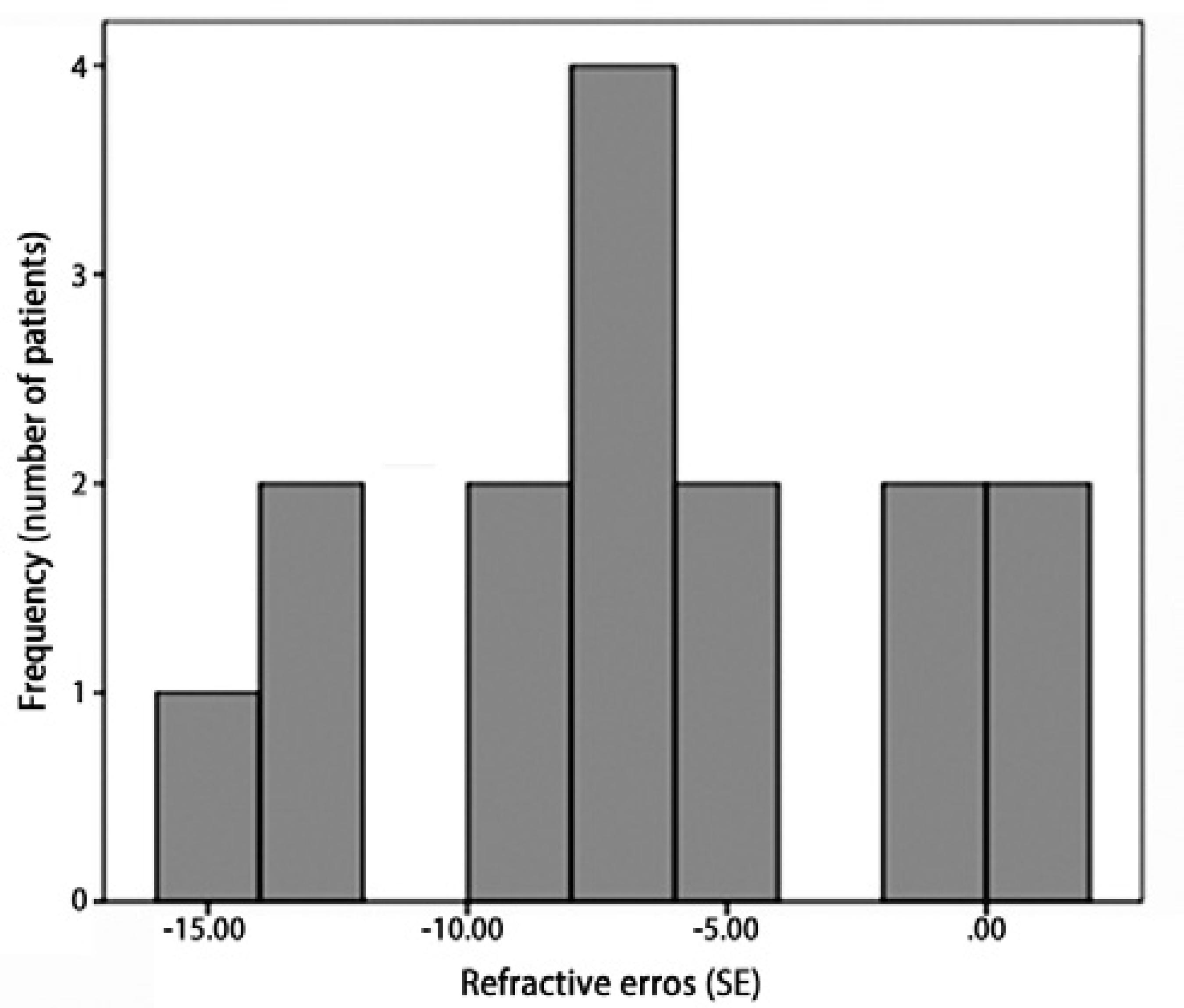
Retinal examinations at an adjusted age of 2 years demonstrated successful retinal vascularization in group I and stable retinal findings in group II. According to refractive analyses at an adjusted age of 2 years, mean SE values were 0.42 ± 3.42 D (range, -8.75 to +5.00 D) in group I and -6.66 ± 4.96 D (range, -15.5 to +1.75 D) in group II (p=0.001, Figure 1). Myopic refraction was significantly greater in group II (86.7%) compared to group I (40%, P=0.010, Figures 2 and 3). In logistic regression analysis, putative risk factors for myopia were evaluated, and laser treatment was identified as significantly associated with the development of myopic refraction (Table 2).
Table 2 Logistic regression analysis of risk-factors for myopia
| 95 % confidence intervals | ||||
|---|---|---|---|---|
| P-value | Odds ratio | Lower | Upper | |
| Laser treatment | 0.011* | 10.558 | 1.720 | 64.790 |
| Gender | 0.287 | 0.437 | 0.095 | 2.000 |
| Gestational age | 0.979 | 0.988 | 0.409 | 2.386 |
| Birth weight | 0.902 | 1.000 | 0.994 | 1.005 |
| Zone involvement | 0.991 | 1.008 | 0.224 | 4.535 |
*= P<0.05.
The incidence of refractive anisometropia was significantly higher in group II (10 children, 66.7%) than group I (5 children, 20%, P=0.009). Further, a significantly higher rate of strabismus was observed in group II (6 children, 40%) compared to group I (2 children, 8%, P=0.036).
DISCUSSION
Several previous studies have evaluated clinical outcomes following ablative therapy for ROP. These studies demonstrated laser photocoagulation results in better anatomic results and causes lower degrees of myopia than cryoablation(17-19). However, laser treatment for APROP has been shown to be associated with a range of unfavorable anatomic and functional outcomes depending on disease severity(2,3,20). The introduction of IVB to ROP therapy has allowed the majority of cases of APROP to be salvaged with successful anatomic outcomes(6,9,21). In recent years, a number of studies have assessed refractive outcomes following IVB treatment and demonstrated IVB in ROP is associated with a lower rate of refractive disorders compared to laser ablation(10-13). We therefore aimed to evaluate 2-year clinical outcomes following IVB monotherapy for APROP in infants at a referral center in Turkey.
Intravitreal bevacizumab has been utilized as monotherapy and as a supplement to laser ablation for APROP and provides successful and prompt disease regression without serious complications(6,22,23). We demonstrated total disease regression with successful anatomical outcomes in all infants who received IVB as a single treatment modality for APROP (Figure 4). Further, there have been a number of reports of disease recurrence after 54 weeks of PMA following IVB injection indicating a need for longer follow-up until at least 70 weeks of PMA(24,25). It has also been suggested that aggressive cases may require additional IVB injections to prevent disease recurrence(6,25). Two patients required additional IVB treatment due to disease recurrence with vascular engorgement and neovascular proliferation at approximately 40 weeks of PMA. Re-injection of IVB was performed in these cases with peripheral retinal vascularization achieved without any complications observed at follow-up at a mean PMA of 79 weeks. On the other hand, laser treatment is a highly destructive procedure in APROP in which the majority of retinal tissue is ablated preventing the growth of vessels peripherally from the point of treatment. Further, a risk of retinal detachment has been reported even after successful laser treatment in such cases(2,16). We observed 2 eyes with Zone I APROP that progressed to stage 4A retinal detachment following laser ablation, which remained stable during the study follow-up period.
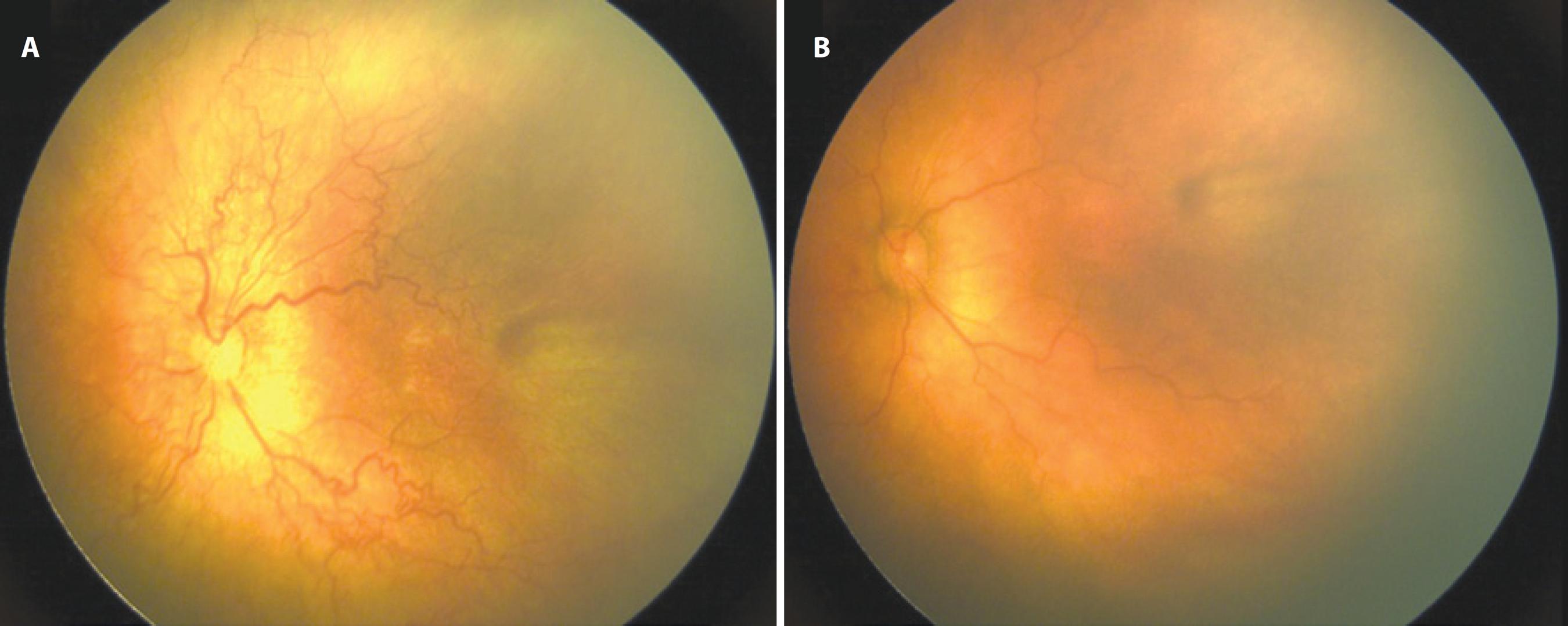
Figure 4 Retinal images of a case of zone I aggressive posterior retinopathy of prematurity (APROP) (A) before and (B) 2 weeks after the injection of intravitreal bevacizumab (IVB). Prominent plus disease and extensive shunt vessels were clearly observed before IVB injection (A). Plus disease resolved with revascularization within zone II 2 weeks after IVB injection.
The presence of systemic detrimental effects following IVB in premature infants remains controversial(26) with no strong evidence of such adverse effects reported to date. In a large cohort study evaluating IVB as a monotherapy in all forms of ROP, the authors posited a positive effect of IVB on systemic parameters in infants, such as early recovery from oxygen dependency and early improvement in nutritional status according to clinical observations(6). No systemic abnormalities following IVB were observed in the current study, at least according to clinical observations. Larger controlled studies are required to fully elucidate the association between IVB and early and long-term systemic side-effects in ROP therapy.
Laser treatment has been well-described as a cause of advanced refractive errors in severe cases of ROP(27,28). Conversely, studies have reported IVB treatment leads to lower degrees of myopic refraction than laser ablation. SE values of -1.04 D and -0.98 D have been reported in 1- to 2-year-olds in some studies(10-12). The BEAT-ROP group recently reported refractive outcomes following IVB therapy in infants with a mean age of 2.5 years. This study demonstrated a mean SE of -1.51 D and -0.58 D following IVB therapy, and -8.44 D and -5.83 D following laser treatment, for Zone I and posterior Zone II ROP, respectively(11). These previous studies and the present study observed lower SE values following IVB therapy (0.42 D) compared to laser photocoagulation therapy (-6.66 D). The development of higher refractive errors following laser ablation is well-known. Studies have shown laser treatment of ROP is associated with shallower anterior chambers and thicker lenses in addition to higher degrees of myopic refraction(29). Contrary to this finding, it has been postulated that the development of retinal vasculature following IVB therapy may maintain ocular growth factor expression leading to normalization of anterior segment development and decreased myopia(11). However, further studies including ocular biometric assessments are required to fully evaluate this relationship.
Anisometropia and strabismus are the predominant risk factors for the development of amblyopia in children following laser ablation for severe ROP(28-30). The present study demonstrated significantly higher rates of refractive anisometropia and strabismus following laser treatment compared to IVB monotherapy. However, as central nervous system abnormalities are commonly observed in premature infants, the relationship between strabismus and IVB therapy should be confirmed by studies identifying related risk factors in larger cohorts.
Limitations of the present study include its retrospective design, the lack of ocular biometry, and the low number of subjects. However, we were able to demonstrate IVB monotherapy was associated with significantly lower degrees of myopic refraction and a decreased incidence of refractive anisometropia and strabismus compared to laser ablation in infants with APROP. Larger cohort studies from different geographical regions with long-term follow-up are required to validate the efficacy of IVB monotherapy for the treatment of APROP.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

