Introduction
Ocular surface tumors encompass malignant, premalignant, and benign lesions arising from the conjunctiva, limbus, or cornea. These neoplasms may originate mainly from squamous epithelia, melanocytes, or lymphocyte cells(1).
Clinical examination of the tumors based on slit-lamp biomicroscopy by a trained professional frequently yields a correct diagnosis, if the clinician is familiar with the clinical characteristics. However, in some instances, only a broad differential diagnosis is possible, and slit-lamp biomicroscopy cannot reliably exclude uncommon diagnosis such as amelanotic malignant melanoma, highlighting the importance of acquiring a clinical diagnosis before administering a treatment. The gold standard is obtaining a biopsy, either incisional or excisional, for histopathology. The main risk of clinical misdiagnosis of an excised benign lesion is exposing the patient to unnecessary surgery; to prevent this, adjunctive diagnostic tests can be performed(2).
Diagnosis may be improved by cytological examination, high-resolution anterior segment ultrasound (UBM), in vivo confocal microscopy, and optical coherence tomography. Cytological sampling is a relatively noninvasive method and is thereby preferable when treatment with nonsurgical techniques such as administration of a topical chemotherapeutic agent with an antineoplastic drug is considered(3). It may also assist in evaluating lesions in cases where surgery may not be appropriate, including patients not medically fit for surgical biopsy(4). In 1954, Larmande and Timsit were the first to use cytodiagnosis in ophthalmology to assist in the evaluation of tumors of the sclerocorneal limbus(5).
Ocular surface cytology can be performed by several methods, including spatula scraping, brush cytology, and impression cytology (IC). IC is a well-established technique for collecting superficial epithelial layers by applying collecting devices (either cellulose acetate filter papers or Biopore membrane device), so that cells adhere to their surface and are removed from the eye to be processed further for analysis by various appropriate methods. IC was first developed to diagnose dry-eye status, and it is now used to diagnose various ocular surface disorders, including neoplasia. It represents a non- or minimally invasive biopsy technique applicable to the conjunctiva, cornea, and limbal area for both diagnosis and follow-up after treatment of tumors(6). Because repeated surgical biopsies of suspicious ocular surface lesions may cause complications such as scarring, lid deformity, limbal stem cell deficiency (LSCD), and great discomfort to the patient, IC can assist in the evaluation(4).
The present review examines and updates the published literature on the utilization of IC for the diagnosis and management of ocular surface tumors and discusses the requirement for further investigation on the subject.
IC technique
After a complete ophthalmological examination, including slit-lamp biomicroscopy, IC can be performed according to methods previously described(4). A drop of topical anesthesia is consistently used. Then, the collection of the superficial cell layers of the ocular surface is performed by forceps-assisted application of a membrane with submicroscopic pores, such as MF-Millipore, onto the patient’s lesion. Membranes are often precut in different shapes and sizes for orientation purposes during processing. Most authors agree to using membranes with pore sizes ranging 0.025-0.45 µm. It is essential to consider the pore size because it affects the consistency of cell collection (the larger the pore size, greater the cellularity) and the resolution of the details under the microscope (morphology was better preserved in the smaller pore size papers). The membrane is firmly pressed against the area to be sampled with the aid of a swab or a solid rod for some seconds and then peeled off using the forceps. Whenever needed, more samples can be collected. They are immediately transferred to be fixed in a solution containing glacial acetic acid, 37% formaldehyde, and ethyl alcohol in a 1:1:20 volume ratio, taking care to completely immerse the membranes. After samples have been fixed, different staining techniques can be performed laboratory analysis. The most used stains include periodic acid-Schiff (PAS), hematoxylin-eosin, Gill’s hematoxylin, and Papanicolaou. The cells can be mounted on a slide after fixation and staining ready for interpretation. PAS is used to stain goblet cells and their secretions and hematoxylin as a counterstain to stain epithelial cells. Papanicolaou helps to better interpret the epithelial changes of squamous metaplasia and the distinct nuclear patterns. These stains have also been used together. Although over the last decade several techniques have used IC samples, light microscopy remains the most used method. To evaluate IC specimens by light microscopy, several features are universally evaluated: the morphology of the epithelial cells, the degree of squamous metaplasia, the nuclear to cytoplasmic (N/C) ratio; the density, shape, and PAS intensity of goblet cells present; and the presence of nonepithelial cells, including inflammatory cells, melanocytic cells, and microorganisms. Atypical cells are identified by the presence of nuclear enlargement, hyperchromasia, irregular nuclear outline, and coarse nuclear chromatin and eventually by the presence of prominent nucleoli, under magnifications of 100×, 200×, and 400×. If different types of atypical cells are observed in the same specimen, more severe stage is considered.
Application of IC in the evaluation of lesions of melanocytic origin
Lesions of melanocytic origin are as common as epithelial tumors and include conjunctival racial melanosis, primary acquired melanosis (PAM), secondary melanosis, nevus, and melanoma. Although majority of the melanocytic lesions are benign, some can be malignant; therefore, distinguishing various conjunctival lesions is crucial(7).
The first IC study of pigmented lesions from the conjunctiva was published in 1992(8). A 73% correlation between IC and histopathology was observed in the diagnosis of 24 tumors, of which three were nevi, nine were melanomas, 10 were cases of PAM, and two were cases of secondary melanosis; examples are shown in figure 1. An increased nuclear-to-cytoplasmic (NC) ratio, an irregular nuclear chromatin pattern, the presence of large nucleoli, and the observation of mitosis and anisokaryosis were regarded as cytological features of malignancy in cells containing melanin. When the relative proportion of atypical melanocytes was low, lesions were cytologically diagnosed as premalignant melanosis equivalent to the histological diagnosis PAM with atypia. If cancerous cells were abundant, the diagnosis was suggestive of melanoma. The authors reported that repeated examinations may increase the sensitivity of the cytological technique. Authors stated that although a diagnostic biopsy may remain necessary for determination of the origin and extent of those lesions, recurrent tumors or suspicious areas may be biopsied less frequently using IC, thus reducing the risk of side effects and patient discomfort(8).
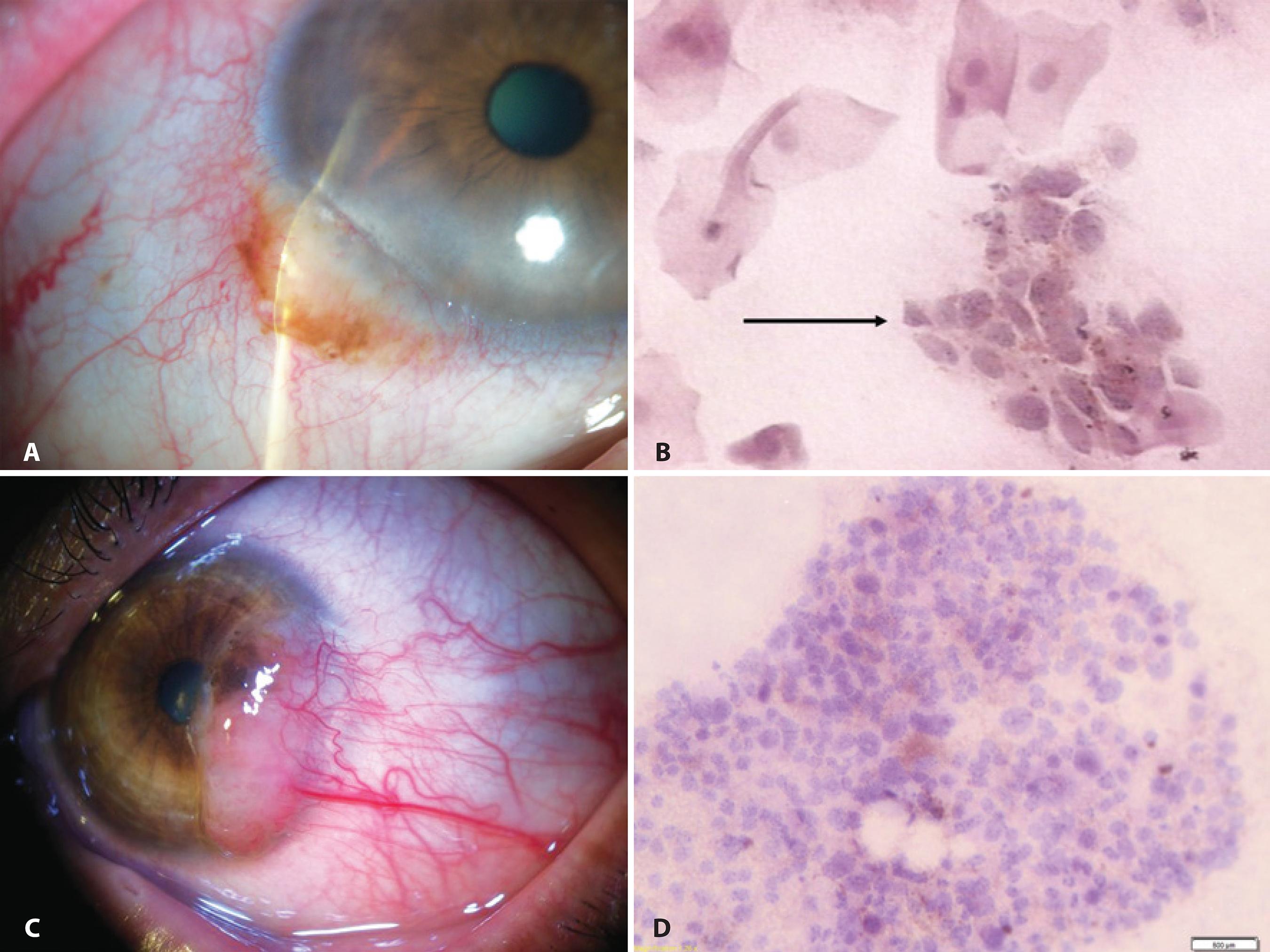
Figure 1 Examples of IC in the evaluation of lesions of melanocytic origin: A) Anterior segment slit-lamp photograph demonstrating a conjunctival nevus. B) IC obtained from the same lesion demonstrating a cluster of nevus cells (arrow) among epithelial cells (Hematoxylin-Eosin staining; original magnification, 400×). C) Anterior segment slit-lamp photograph of malignant melanoma. D) IC obtained from the sample depicted in (C) demonstrated clusters of pleomorphic atypical tumor-dissociated cells with different sizes and anisokaryosis characterized by large and irregular nuclei in a cytomorphology not resembling epithelial cells. Brown melanin granules can be seen inside the cytoplasm of the malignant melanocytes (Hematoxylin-Eosin staining; original magnification, 200×).
In 2007, a study revealed 68 melanocytic conjunctival lesions, of which 31 were nevi, nine were melanoma, and 28 were PAM. The authors compared the Biopore membrane IC (referred to as “Biopore”) with exfoliative cytology (EC) in these lesions. Twenty-three of the 26 samples analyzed by Biopore and 20 of the 24 samples analyzed by EC correlated with the corresponding histology. Biopore accurately predicted the outcome in 88% and EC in 83% of the lesions. The authors concluded that Biopore could be used in cytology of melanocytic lesions and was easier and faster to interpret than EC. If difficult with Biopore, sampling of the fornix, caruncula, and ocular material in children could be performed by EC. Because some melanocytic lesions will be covered with one or more layers of normal epithelium, cytology could only provide a realistic picture of a lesion when it was able to sample deeper than the most superficial layer of epithelial cells. Biopore, however, may sample only the first layer of cells on the conjunctiva, unless it is repeated several times to acquire cells of deeper layers(9). Similarly, IC with cellulose acetate filters was able to sample deeper layers when performed repeatedly(10).
A case of a patient with an irregular pigmented lesion of the lower eyelid margin simulating malignant tumor, which was treated based on the results of IC and diagnosed with secondary melanosis by histology, was presented in 2009. The importance of IC was emphasized as an effective and safe method circumventing unnecessary and extensive procedures(11).
A few melanocytic lesions, including four nevi and one melanoma, were examined in another study, and for such cases, results of both IC and histopathological features correlated(5).
IC features of 35 conjunctival nevi from children and adults referred to as more noticeable were described in 2009. Approximately 26% were amelanotic but could be identified as localized areas of hyperemia. Using criteria derived from histology, IC was reported for conjunctival nevi when nests or clusters of nevus cells were observed within the epithelium layer containing or not containing mucous-secreting goblet cells. Epithelial cell layers demonstrated normal morphology, or, when the lesion was elevated, showed signs of squamous metaplasia (SM). IC confirmed the clinical diagnosis by demonstrating typical histopathological features of the superficial layers of conjunctival nevi in 91.4% of the cases. For amelanotic nevi, IC also allows differential diagnosis from other non-pigmented lesions(12).
Recently, a case of an amelanotic corneally displaced malignant conjunctival melanoma was described. The authors showed that IC performed prior to the treatment provided the first clue for the diagnosis later confirmed by histopathology. IC samples revealed abundant clusters of pleomorphic atypical tumor-dissociated cells with different sizes and anisokaryosis characterized by large and irregular nuclei with occasionally prominent nucleoli in a cytomorphology not resembling epithelial cells. Some of the atypical cells were spindle-shaped. Melanin pigment was absent. A few nonneoplastic squamous epithelial cells were also observed. Clinical diagnosis of amelanotic melanoma is challenging, and IC can assist in supporting the initial diagnosis when interpreted by a trained cytologist or under guidance of an ocular pathologist. For amelanotic melanoma, IC enables differential diagnosis from other nonpigmented lesions(13). In addition, incisional biopsy of melanoma should be avoided because of the risk of local tumor dissemination(14).
Application of IC in the evaluation of lesions of epithelial origin
Ocular surface squamous neoplasia (OSSN) is the most common tumor of the ocular surface. The spectrum of OSSN ranges from mild to severe dysplasia, through full-thickness epithelial involvement, to invasive squamous cell carcinoma (SCC). Although the clinical appearance of a lesion can be suggestive of OSSN, tissue biopsy is necessary to confirm the diagnosis because the different stages of OSSN are extremely difficult to distinguish by slit-lamp biomicroscopy, with an accuracy of clinical diagnosis by experienced clinicians of approximately 40%(3).
It has been reported that IC immunostained with cytokeratin antibodies and HMB-45 was useful to differentiate a pigmented conjunctival seborrheic keratosis masquerading as malignant melanoma. IC disclosed basaloid cells intermixing with squamoid cells, and these cells demonstrated positive immunoreactivity to cytokeratin and no reactivity to HMB-45 and therefore were proven to represent an epithelium-derived tumor despite of being pigmented. This report illustrated that IC combined with immunocytochemical staining may be a valuable diagnostic aid in the differentiation of pigmented conjunctival tumors prior to treatment(15).
The published correlation rate with IC for predicting the subsequent histological findings ranged between 77% (55/71) and 80% (20/25), and both cellulose acetate(16) and Biopore membranes(17) have been successfully used. The difficulty in interpreting these IC specimens caused by the paucity of published criteria was overcome with the publication by Nolan et al., who described in detail the cytomorphology of OSSN based on a high number of cases. The following cytological criteria were used to diagnose intraepithelial OSSN: nuclear enlargement (more than two times the dimensions of the nucleus of normal conjunctival cells), presence of irregular nuclear contour, coarsely clumped chromatin, nuclear pleomorphism, binucleation or multinucleation, and evident nucleoli. When nuclear enlargement was less than twice the dimensions in normal conjunctival cells or when it was limited to only few squamous cells, the specimen was categorized as having atypical squamous cells indefinite for dysplasia. If none of the abovementioned characteristics was observed, the specimen was regarded negative. The finding of syncytia-like groupings, intraepithelial infiltration of inflammatory cells, and macronucleoli may be suggestive of SCC in some samples(18). Nevertheless, at present, no unique specific cytological feature to differentiate SCC from intraepithelial lesions in IC specimens has been identified. According to these reports, there were no false-positives identified by IC(16-19). Examples are shown in figure 2.
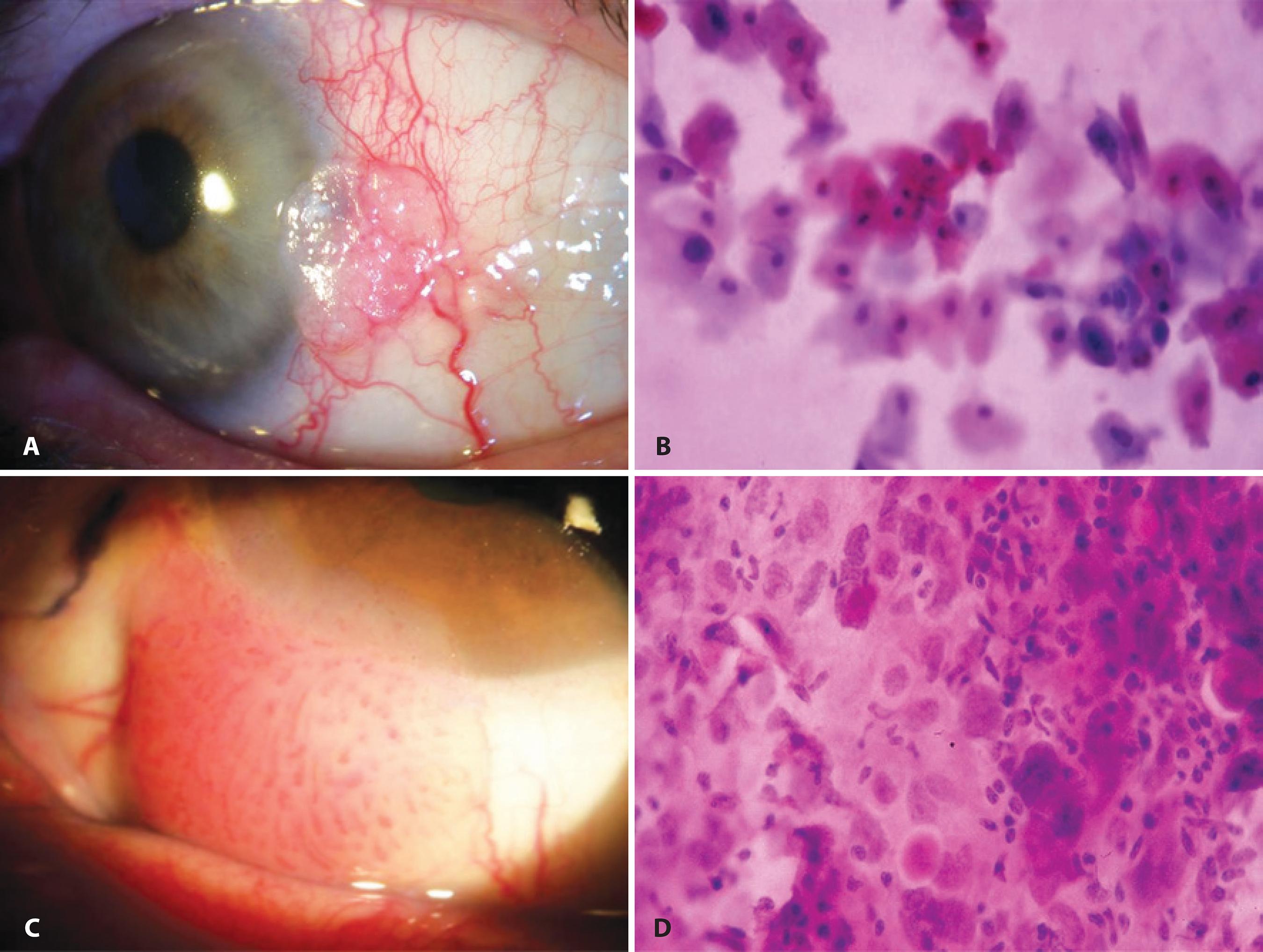
Figure 2 Example of IC in the evaluation of lesions of epithelial origin (ocular surface squamous neoplasia): A) Anterior segment slit-lamp photograph demonstrating conjunctival intraepithelial neoplasia. B) IC obtained from this lesion demonstrating atypical epithelial cells with mild nuclear enlargement, anisokaryosis, and remarkable hyperchromasia (Hematoxylin-Eosin staining; original magnification, 400×). C) Anterior segment slit-lamp photograph of invasive squamous cell carcinoma of the conjunctiva. D) IC demonstrating atypical epithelial cells showing nuclear enlargement, marked increase in the nuclear-to-cytoplasmic ratio, anisokaryosis, hyperchromasia, and a syncytial-like arrangement with absence of well-defined cytoplasmic borders (Hematoxylin-Eosin staining; original magnification, 400×).
Notably, the cytology of subclinical intraepithelial OSSN has already been described. The cytological pattern for OSSN with no clinically visible abnormality differed from that observed in the eyes with clinically detectable disease; there were often a few dysplastic cells lying within sheets of normal epithelium(18).
In 2002, Chan et al. showed that IC obtained from surface cells overlying a pterygium was abnormal, typically exhibiting SM with increased goblet cell density. Altered cytology could also be demonstrated in the inferior bulbar conjunctiva and interpalpebral conjunctiva, without clinical evidence of pterygium. This suggested a graded series of changes occurring throughout the bulbar conjunctiva, with the most advanced occurring directly over the pterygium, confirming that it was indeed an ocular surface disorder(20).
A case of conjunctiva-cornea intraepithelial neoplasia (CCIN) treated with topical mitomycin-C (MMC) and interferon alfa-2b in cycles was described in 2003. The patient was referred for LCSD and epithelial defect but IC specimens were suggestive of CCIN. After differentiation from LCSD by dye staining and IC, the patient was successfully treated(21).
Another study found that IC had a positive and negative predictive value of 97.4% and 53.9%, respectively, when compared with histology(5).
In 2009, Barros et al. described an index score modified from the Bethesda system for reporting cervical cytologic diagnoses to differentiate SCC from pre-invasive ocular surface lesions by IC (n=39). They revealed a predictive index score of ≥4.25 representing the best cut-off point for SCC with a sensitivity of 95%, specificity of 93%, positive predictive value of 95%, and negative predictive value of 93%(4). Four of seven parameters included in their regression model (nuclear enlargement > three-fold, syncytial-like groupings, increased NC ratio, and indistinct cytoplasm border) were visible using clinical confocal microscopy (CCM). One parameter (prominent nucleoli) is currently undetectable by CCM. The last two parameters (cellular hyperchromasia and eosinophilic cytoplasm) would require specific stains unavailable in vivo. The introduction of in vivo stains or biomarkers to better visualize these cellular details would be useful to improve image quality and to obtain more detailed information. A novel CCM specific index score to differentiate SCC from preinvasive ocular surface lesions is still necessary(22).
IC may be less sensitive for cases with keratotic lesions because an abundance of surface keratin can make sampling inaccurate(5). To minimize this problem, authors have recommended collecting at least two samples over the same area from a suspicious lesion(4,23). For diagnosing OSSN, adding a second and a third evaluation of IC provided significantly more sensitivity than including only one(23). Nevertheless, it should considered that IC is very helpful, unless the result conflicts with the clinical scenario or when the actual clinical diagnosis is uncertain and the result is negative. In these cases, surgical biopsy needs to be performed for accurate diagnosis(5,23).
In the study by Ballalai et al., 0.02% topical MMC was used to treat patients with OSSN. Before the treatment, cytology showed the presence of neoplastic cells in patients with primary tumors, avoiding surgical biopsy and treatment delay(24).
A great advantage of using IC is the preservation of limbal stem cells, responsible for renewal of corneal epithelium throughout life. In most OSSN cases, the lesions affect predominantly the limbus and have a tendency to recur. IC offers a safer tool for diagnosis than repeated biopsy(4). Moreover, IC can be used during post-surgery follow-up to identify any recurrence of the disease as well as the effects of topical treatment such as chemotherapy with antineoplastic drugs like MMC(25).
Application of IC following tumor treatment
Treatment for OSSN has historically been surgery but nonsurgical interventions have also been adopted. Adjunctive therapies allowed the treatment of subclinical disease at a site different from that of the clinically evident tumor. Nevertheless, topical chemotherapeutic drugs can be potentially toxic to the ocular surface(26). In 2001, IC was used to study the effects of topical MMC in the treatment of OSSN; 0.04% MMC induced cell death mainly by apoptosis or rarely by necrosis and changes induced in the ocular surface persisted for at least 8 months. MMC induced cytomegaly, cytoplasmic vacuolation, nucleomegaly with nuclear wrinkling, and binucleation or multinucleation. The N/C ratio in these enlarged cells was normal. These changes mimicked those observed following radiation therapy in uterine cervical cancer. Nuclear and cell size increased along with increasing N/C ratio in some dysplastic cells(25). Yamamoto et al. used IC during diagnosis and follow-up, resulting in successful treatment with 5-fluorouracil of an intraepithelial OSSN with LSCD that was refractive to topical MMC(27).
Dogru et al. evaluated the tear function and ocular surface alterations in patients with primary intraepithelial OSSN before and after treatment with 0.04% topical MMC. Initial IC specimens showed loss of goblet cells, higher grades of SM, and areas of isolated keratinized, binucleated, and actively mitotic disfigured epithelial cells in all patients. The mean goblet cell density and SM grade were observed to having significantly improved at the last visit of the patients. IC proved useful in attaining the diagnosis of OSSN, evaluating the effect of treatment and showing MMC-related long-term changes on the ocular surface(28).
In 2005, Prabhasawat et al. reported complete tumor regression observed clinically and by IC, demonstrating the efficacy of 0.002% topical MMC as an adjunctive and alternative treatment in primary and recurrent OSSN; IC exhibited tumor-free specimens with cellular elongation as a result of chemotherapy(29).
Notably, cytological changes mimicking malignancy have been reported in conjunctiva up to 6 weeks following topical MMC therapy. Nevertheless, there are features which help to differentiate these changes: epithelial cells affected by the drug show a proportionate increase in both cytoplasm and nucleus, preserving a normal NC ratio (cytomegaly), unlike the case of increased NC ratio (cariomegaly) in OSSN. The distinction of MMC-related changes from OSSN cells in IC specimens can be performed when the cell border is clearly visible and the N/C ratio can be estimated. Differentiation becomes difficult in cells with large hyperchromatic nuclei where the cell outline is not clearly defined because of overlapping cells or attenuation of the vacuolated cytoplasm. Therefore, studying such cells for which cell size can be clearly assessed is crucial(25).
Westekemper et al. examined ocular surface integrity of ten patients with large and diffuse conjunctival melanoma who underwent proton beam radiation. The IC revealed conjunctival SM in nine cases, indicating a radiogenic, persisting disturbance in the differentiation of the conjunctival epithelial cells. The tear film instability correlated with goblet cell loss and meibomian gland dysfunction(30).
The use of topical MMC has been described by some authors not only for OSSN but also for melanocytic lesions such as PAM with atypia. However, its prolonged use may be associated with a high incidence of complications like LSCD. IC diagnoses ocular surface lesions and also evaluates possible local side effects following treatment. Five cases of proven LSCD by IC resulting as a complication of topical treatment with MMC for PAM with atypia have been reported(31).
Rodríguez Feijoo et al. reported that making an accurate differential diagnosis between keratoacanthoma and SCC by histology as well as carrying out close monitoring after surgery due to the possibility of relapse and conversion to SCC is important. Therefore, they proposed the use of IC as a method for monitoring such patients. After the treatment, IC exhibited large altered epithelial cells with intracellular union changes and an NC ratio of 1:20. A second series of IC tests performed 3 months after the first series showed the same results(32).
Recently, Faramarzi and Feizi evaluated the efficacy of perilesional/ subconjunctival injections of an antivascular endothelial growth factor, bevacizumab, for treatment of a group of 10 eyes with primary OSSN. Based on clinical presentation and IC results, they showed that the treatment was effective in terms of decreasing the size of conjunctival OSSN when the lesion was limited to the conjunctiva. However, this therapy had no effect on corneal extensions of the OSSN(33).
Application of IC in the evaluation of tumors of sebaceous origin
In 2003, Sawada et al. demonstrated that IC detected conjunctival intraepithelial invasion from sebaceous cell carcinoma of the eyelid in four patients with severe unilateral blepharoconjunctivitis. IC showed numerous inflammatory cells and abnormal tumor cells with atypia and characteristic cytoplasmic vacuoles, consistent with dissolved sebaceous contents(34-35). They represented areas where lipid was contained before it was dissolved by alcohol; an example is shown in figure 3. The diagnosis was confirmed by histology from full-thickness wedge resection of the eyelids. When pagetoid spread in advanced cases of sebaceous cell carcinoma results in a superficial or full-thickness replacement of the normal conjunctival epithelium with tumor cells, the superficial abnormal cells can be detected by IC. However, areas on the conjunctiva with pagetoid spread may exist without full-thickness epithelial disease. In such cases, IC may sample only the superficial normal epithelial cells and may fail to detect the tumor cells concealed in the deeper layers. Because sebaceous carcinoma can masquerade as several benign conditions such as blepharitis, the investigations should include IC and biopsy in cases that are not responsive to medication. If cellular atypia is present, a full-thickness lid biopsy should be performed(34).
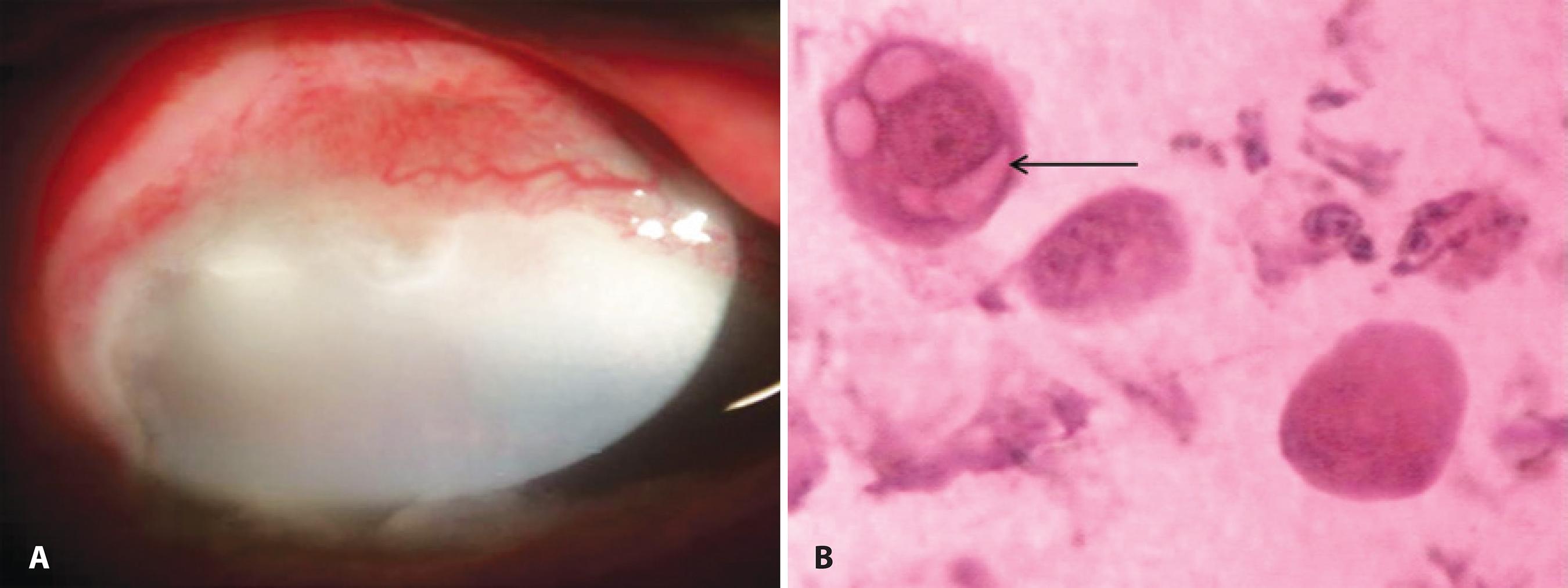
Figure 3 A) Anterior segment slit-lamp photograph demonstrating conjunctival intraepithelial invasion from a sebaceous cell carcinoma of the eyelid. B) IC showing inflammatory cells and a tumor cell with atypia, abnormal prominent nucleoli, and characteristic cytoplasmic vacuoles (arrow) consistent with intracellular dissolved sebaceous contents. These cells were PAS-negative, suggestive of non-goblet cell origin. No evident goblet cells were observed in the epithelium (PAS and Hematoxylin-Eosin staining; original magnification, 400×).
Application of IC in the evaluation of ulcerative eyelid malignancy
Thirty-two histopathologically proven malignant eyelid lesions diagnosed over a 2-year period, including 13 basal cell carcinomas, 11 sebaceous carcinomas, four SCC, two malignant melanomas, and two poorly differentiated carcinomas, formed the study group described very recently. The results of IC were compared with those of obtained by histopathological analysis in the study group and with an age- and sex-matched group of benign cases as controls. The sensitivity of IC was 84% (27/32) for the diagnosis of malignancy and 28% (9/32) for categorization of the type of malignancy. Because of its low sensitivity in terms of cytological categorization of the type of malignancy, IC cannot be recommended in the primary diagnosis of eyelid malignancies. Nevertheless, with experience and improvement in the technique, it may prove to be a useful tool in deciding future management, particularly in recurrences of histopathologically confirmed eyelid malignancies, where biopsies may be avoided(36).
IC , imaging, and histopathology
Clinical examination is subjective, is unable to assess cellular morphology, and may not detect subclinical microscopic diseases. A surgical biopsy to confirm the resolution of an OSSN could miss small residual lesions. Thus, an incisional biopsy may miss lesions that were not included in the excised tissue. The biopsy is based on clinically visible disease and may produce false-negative results. The false clinical impression of tumor resolution can result in premature termination of topical treatment and an increased risk of recurrence. These lesions can spread along the basal conjunctival layers far beyond the clinical lesion, and thus may be missed clinically. Excisional biopsy, despite being the most traditional and accurate means, may induce conjunctival scarring, LSCD, and visually disturbing corneal scarring. Due to the multifocal nature of OSSN, surgical excision results in extensive collateral damage to adjacent areas of normal epithelium(37).
In addition to IC, newer diagnostic techniques including CCM(22), toluidine blue(38), and ultra-high resolution anterior segment optical coherence tomography (UHR-OCT)(39) have been reported to aid in the diagnosis of OSSN. All these techniques have limitations and require skilled professionals to perform the tests and interpret the results. IC assesses only superficial layers of cells, which are not always representative of deeper layers, whereas CCM does not provide cross-sectional views hence not being useful for determining the vertical and horizontal extent of the lesion. Therefore, ensuring that the exact same area of the ocular surface is analyzed by CMM at follow-up examinations can be challenging. Regarding UHR-OCT, lesions, which are thickly pigmented lesions or show leukoplakia, tend to impede the penetration of light to deeper tissues, impairing the determination of the posterior limit of the lesion. Optical information at the time of the study was not sufficient to study signs of cellular atypia and was not able to rule out microinvasion(37). Similar to UHR-OCT, IC may not distinguish in situ from minimally invasive disease(6).
Each imaging modality has the advantage of being noninvasive, and each has been is useful in the detection of OSSN. However, both UBM and some confocal microscopy devices require contact with the ocular surface, increasing both the length of time and technical expertise required for their performance. Furthermore, although confocal microscopy has the advantage of detailing individual cell morphology, which is currently outside of the capability of UHR-OCT, it targets a very limited area. OCT has the advantage of higher-resolution images, but shadowing may occur in thick lesions or those with leukoplakia. UBM has greater depth of penetration but lower resolution and cannot evaluate the epithelial versus subepithelial nature of a lesion. No data are available regarding inter- and intraobserver variability for the assessment of ocular surface pathology using the UHR-OCT(40). Despite UHR-OCT having the advantage over IC of providing relatively deeper scans of the entire epithelium and the underlying tissue, it cannot reliably detect invasion(37). In addition, UHR-OCT machines are largely limited to academic institutions(39).
IC may be an inexpensive tool that can be used in the outpatient clinic setting to help provide an objective evaluation of suspicious lesions that enables patients to make better informed decisions regarding the treatment requirements. Results of IC may also help the ophthalmologist decide whether incisional or excisional biopsy should be performed and whether any other associated procedures, such as freeze-thaw cryotherapy of the sclera/limbus and/or ethanol application to the cornea, are required. IC provides a flat mount of an area as large as the size of the applied filter paper with well-preserved morphology. In comparison, conjunctival smears destroy much of the morphological information and conjunctival biopsies provide information from a relatively small sample of the surface epithelium, both because of the difficulty of preparing flat mounts and because of their small sizes. Therefore, IC is ideal for sampling the corneal epithelium(40).
FINAL COMMENTS
OSSN masquerades as scar tissue or pannus; in addition, it can appear in association with pterygia(3). Thus, the question of using IC for the detection of OSSN in the setting of concomitant ocular surface disease requires further studies. Recently, Barros et al. reported that IC demonstrated high agreement with the results of the histopathological analysis for detecting atypical epithelial cells from unsuspected OSSN in cases of pterygia from Brazil, showing unsuspected and associated OSSN cells in 13 specimens (40%)(41).
IC presents great advantages: (1) it provides a source of intact and well-preserved epithelial cells from the ocular surface in any type of ocular surface pathology; (2) it is a nonsurgical, easy-to-perform, quick, and inexpensive technique that can always be performed on an outpatient basis; (3) only topical anesthesia is required, and no side effects or contraindications have ever been noted and thus it can be applied to children; (4) repeated IC sampling in the same patient over time is an excellent way to demonstrate changes due to a certain event, to monitor the progress of a disease, or to follow the effect of a therapeutic intervention; (5) IC maintains cell-to-cell contacts, preventing the problems of EC or brush cytology, which may destroy much of the cell morphology, cause overlapping of cells, and hamper clear visualization of the in vivo arrangement of the cells; (6) IC samples can be processed using any type of microscopy in addition to polymerase chain reaction (PCR), immunoblotting analyses, and/or flow cytometry. Based on all these advantages, IC has become the technique of choice for sampling ocular surface epithelium for being a very useful research tool in both basic and clinical aspects(41-42). Although IC cannot replace histology, it has an important role in the diagnosis and management of patients with OSSN in a less invasive manner. A tool such as IC that aids the diagnosis of OSSN is of particular relevance to Brazilian patients, who live in a country closer to the equator line, with a climate and an ultraviolet-B light index that may contribute to the appearance and development of such tumors in its population. The correlation between sun exposure and OSSN has been well established(43). The importance of IC lies in its capacity to detect both the presence and extent of OSSN when the clinical diagnosis is difficult, to detect subclinical disease and follow up on previously diagnosed disease(4,18). Expertise in IC is acquired by continuing experience including close reviews, correlation with all possible subsequent histology specimens, and clinicopathological correlation. This enables the cytologist to gain familiarity and become aware of the eventual difficult areas such as keratinizing lesions(17).
Because IC has not presented sensitivity and specificity of 100%, the prospective use of the Barros score for predicting SCC needs to be further evaluated using a large number of patients(4). The development of a novel immunohistochemical analysis with a proliferative index such as that for Ki-67 could aid in IC specimens becoming a diagnostic marker for OSSN and in obtaining prognostic information regarding the risk of recurrence in a manner similar to the current use of histology(44). This combination of IC and immunocytochemistry was first described by Krenzer and Freddo in normal human conjunctiva in 1997, enabling the simultaneous evaluation of IC specimens for immunoreactivity to cytokeratin and morphological details(45). Nevertheless, as indicated in this review, there was only a single case(15) using this combined technique in the evaluation of an ocular surface tumor. Thus, the sensitivity and reliability of IC combined with immunocytochemical staining in the differentiation of ocular surface tumors need further evaluation in large-scale studies.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

