INTRODUCTION
Keratoconus is a non-inflammatory ectatic corneal disorder. It is characterized by paracentral corneal thinning and increased corneal curvature leading to irregular astigmatism, myopia, and protrusion(1). Initial treatment includes using spectacles and rigid contact lenses. Several surgical procedures, such as corneal transplantation, epikeratophakia, and photorefractive keratectomy, have been developed for more advanced cases; however, some of these have yielded disappointing results(2-4).
Intrastromal corneal ring segments (ICRS) are an interesting alternative for keratoconus treatment in patients with clear corneas and contact lens intolerance(5-7). There are several different models with varying sizes and arch thicknesses. These segments induce an arch shortening effect in the lamella leading to central flattening of the cornea. The main advantages of this procedure are its safety, reversibility, and stability as well as the fact that segments do not affect the corneal visual axis(8-13). The intrastromal tunnel for ring implantation was initially manually constructed; however, complications, such as epithelial defects, depth asymmetry, and perforation, were reported(5,6,13).
Femtosecond laser has recently been used to create the tunnel for ring implantation. This technique reportedly creates a tunnel with precise depth, width, and location leading to minimal haze and edema as well as minimal surgical complications. The laser acts via photodisruption and can be programed to create tunnels for segment placement at predetermined depths. Studies have shown that tunnel creation using this technique is easier, more precise, and more predictable than the technique involving a conventional mechanical microkeratome(7).
We studied the outcomes of patients with keratoconus who underwent intrastromal corneal ring implantation (ICRSI) using femtosecond laser (Ziemer LDV) at the Hospital de Olhos do Paraná. Our aim was to evaluate the visual improvement in these patients, the safety of this technique, and the differences between ICRSI alone and its combination with postoperative ultraviolet A riboflavin-mediated corneal collagen crosslinking (CXL) procedure.
METHODS
This was an observational study approved by the Research Ethics Committee of Positivo University on November 22, 2013 under the protocol number 465.316.
The medical records of patients with keratoconus seen at Hospital de Olhos do Paraná who underwent ICRSI using femtosecond laser or who had ICRSI combined with CXL were retrospectively analyzed. The medical records from January 2011 to December 2012 were reviewed, and the time of the follow-up that was considered for the analysis was from 6-15 months.
The inclusion criteria were as follows: (1) eyes with keratoconus in the topographical analysis confirmed by two experienced ophthalmologists; (2) presence of the clear central cornea; (3) corrected visual acuity (VA) of less than 20/40 according to the Snellen chart; (4) intolerance to contact lenses or no improvement in VA with contact lenses; (5) a minimum central corneal thickness of 380 µ and a minimum corneal thickness of 400 µ at the site of the incision and construction of the corneal tunnel for ring implants; and (6) a minimum central corneal thickness of 400 µ for the CXL procedure.
The exclusion criteria were as follows: (1) incomplete clinical data in the medical records; (2) loss to follow-up of patients 3 months postoperatively; (3) history of eye diseases such as glaucoma, cataract, diabetic retinopathy, and age-related macular disease; (4) preoperative plano spherical equivalent (SE); (5) history of eye surgery; and (6) collagen disease or pregnancy.
In this study, ICRSI (CornealRing, Visiontech®, Belo Horizonte, Brazil) was performed by two experienced surgeons using the femtosecond laser (Ziemer LDV, Ziemer Ophthalmic Systems, Switzerland) to create the stromal tunnel. The channel’s inner diameter was set to 4.8 mm and the outer diameter was set between 6.02 to 6.26 mm; the entry cut thickness was set to 1.3 mm and 10 degrees in length (at the steepest topographical axis), the velocity for was set for 3.2 mm/s, 1.0 mm/s and 5.2 mm/s for the stroma, vertical incision and insertion, respectively. The power was set at 100% for the stroma and at 150% for the vertical incision. A vacuum setting at 750 mbar on controlled mode with automatic release mode was applied. The nomogram is available online at www.cornealring.com.
The selected clinical and topographical data were assessed pre-and postoperatively. The clinical examination data included the best spectacle-corrected VA, spherical and cylindrical diopter values, and SE. The topographical data included the maximum, minimum, and mean keratometric values (Kmax, Kmin, and Kmed, respectively) as well as the corneal apex value in diopters (D). In addition, the information on the transoperative and postoperative complications and on the performance of the corneal crosslinking was considered.
The differences in VA and topographical data between pre- and post-ICRSI period as well as the perioperative and postoperative complications were assessed during the study period.
CXL was performed on eyes that showed evidence of ectasia and that still had low VA 3 months after ICRSI. Ectasia was considered if the topography showed at least 0.5 D of progression in the corneal curvature index over a 6-month period.
After central corneal abrasion in the operating room and under appropriate sterile conditions, 0.5% proparacaine hydrochloride drops were used as local anesthesia. The central corneal epithelium was removed (9 mm) with a blunt spatula. A photosynthetic riboflavin 0.1% solution (10 mg riboflavin-5-phosphate in 10 mL of dextran T-500 20%), was applied to the cornea every 5 min for 30 min before the ultraviolet A (UVA) irradiation.
The cornea was then exposed to UVA irradiation with a solid state device: the X-Link (Opto Electronics, San Carlos, Brazil), which emitted light at a wavelength of 370 ± 5 nm at an irradiance of 3 mW/cm2 or 5.4 J/cm2. The corneal exposure lasted 30 min, while the riboflavin solution was applied every 5 min.
The data were described as the mean, median, minimum and maximum values, and standard deviations. The preoperative comparisons were performed using Student’s t-test for independent samples. The postoperative comparisons and differences between pre- and postoperative values were based on the model analysis of covariance (ANCOVA), including the preoperative evaluation as a covariate. To compare the pre- and post-ratings within each treatment, we used Student’s t-test for paired samples; for variable VA logMAR comparisons, we used the nonparametric Mann-Whitney test. Comparisons between pre- and postoperative values within each group were made utilizing the non-parametric Wilcoxon test. P values of <0.05 were considered statistically significant. The normal variables were evaluated by the Kolmogorov-Smirnov test. All analyses were performed using IBM SPSS Statistics v.20.
RESULTS
Thirty-one patients with keratoconus who underwent ICRSI using femtosecond laser (32 eyes) were studied. The group 1 consisted of 14 (63%) men and eight (37%) women with ages between 16 and 52 years (mean 28.9 ± 8.2 years). The group 2 consisted of seven (70%) men and three (30%) women with ages between 19 and 37 years (mean, 27 ± 6.4).
Except for the mean K (p=0.02), all other preoperative variables were similar between the two groups (age: p=0.53; gender: p=1; spherical refractive error: p=0.67; astigmatism: p=0.09; corneal apex: p=0.49; and VA in logMAR: p=0.76), as presented in table 1.
Table 1 Evaluation of associated factors with conus progression after ICRS
| Group | |||
|---|---|---|---|
| Variable | ICRSI | ICRSI + CXL | P value* |
| Age (years) | 28.90 ± 8.20 | 27.00 ± 6.40 | 0.529 |
| Male | 63.6% | 70.0% | 1 |
| Spheric pré | -3.99 ± 3.08 | -4.45 ± 2.18 | 0.674 |
| Cilindric pré | -3.80 ± 1.96 | -4.93 ± 1.03 | 0.098 |
| SE pré | -5.89 ± 3.37 | -6.91 ± 1.93 | 0.379 |
| K-average pré | 50.90 ± 2.75 | 47.06 ± 2.24 | 0.018 |
| Apex pré | 58.19 ± 2.99 | 56.56 ± 4.75 | 0.493 |
| VA logMAR | 0.54 ± 0.24 | 0.56 ± 0.34 | 0.764 |
*Student's t-test or exact Fisher test, p<0.05
CXL was performed in 10 eyes (31%) at a mean time of 5.8 ± 2.04 months after the ring insertion. In all cases in which CXL was performed, there were no complications or need for ring repositioning.
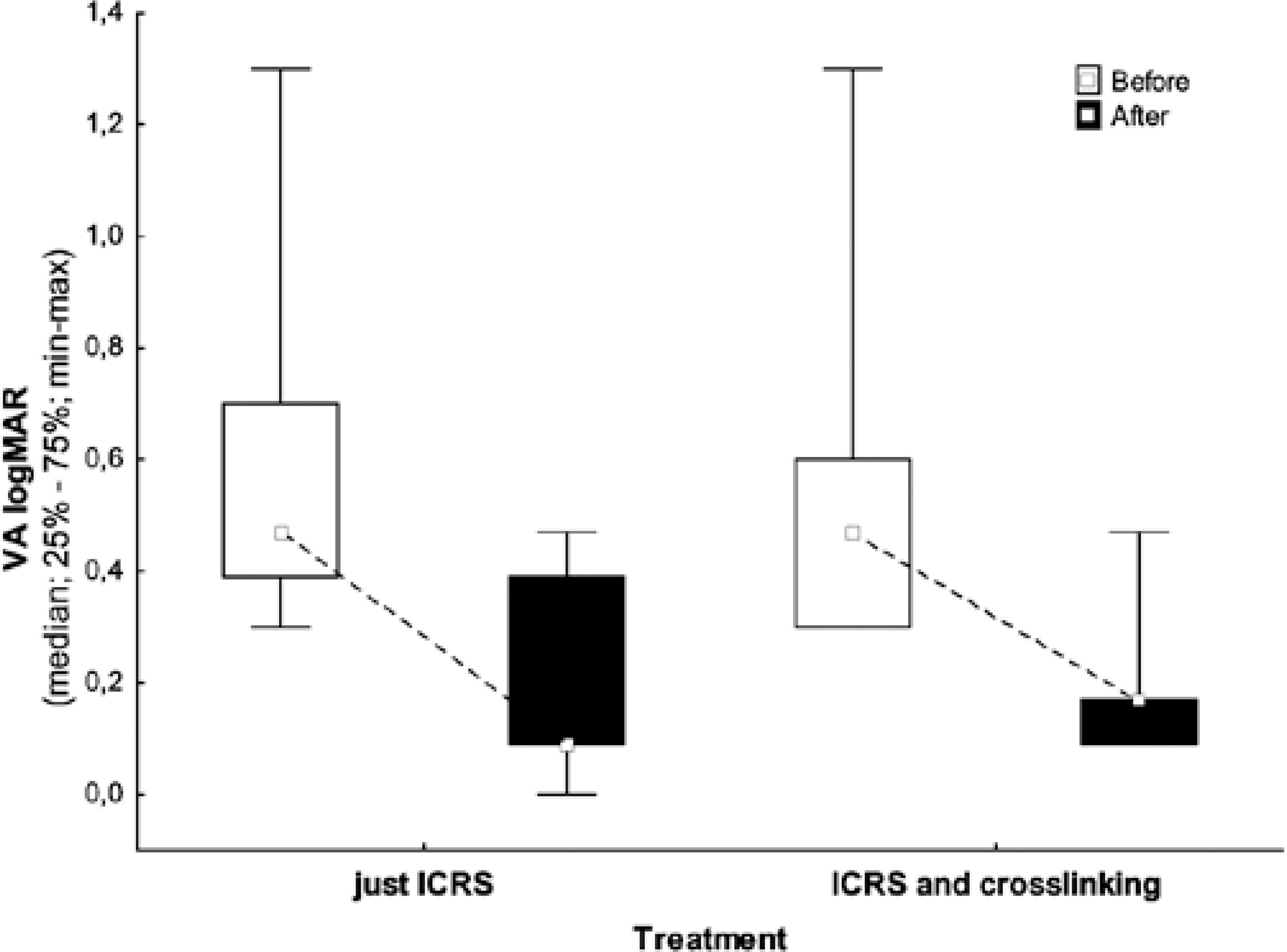
VA of patients preoperatively ranged from 20/40 to 20/400 (Snellen chart) in both groups. No patient postoperatively presented with VA worse than 20/60, and 78% exhibited VA better than or equal to 20/40 with spectacles in both groups. Table 2 and figure 1 show differences in the best-corrected VAs before and after the procedure.
Table 2 Differences in best-corrected visual acuity between groups
| Treatment | n | Mean | Median | Minimum | Maximum | Standard deviation | P value | |
|---|---|---|---|---|---|---|---|---|
| Preoperative VA | ICRSI | 22 | 0.54 | 0.47 | -0.30 | 1.30 | 0.24 | |
| logMAR | ICRSI + CXL | 10 | 0.56 | 0.47 | -0.30 | 1.30 | 0.34 | 0.764 |
| Postoperative VA | ICRSI | 22 | 0.18 | 0.09 | -0.00 | 0.47 | 0.17 | |
| logMAR | ICRSI + CXL | 10 | 0.17 | 0.17 | -0.09 | 0.47 | 0.11 | 0.646 |
| Difference pre- and post-operative | ICRSI | 22 | 0.36 | 0.38 | -0.17 | 0.83 | 0.22 | |
| ICRSI + CXL | 10 | 0.40 | 0.34 | -0.13 | 0.83 | 0.25 | 0.857 |
*= nonparametric Mann-Whitney test, p<0.05. VA= visual acuity; ICRSI= intrastromal corneal ring implantation; CXL= crosslinking.
In the group 1, the spherical refraction ranged from -9.50 D to plano preoperatively, with a mean cylindrical value of -3.80 D. In the group 2, the spherical refraction ranged from -7.00 to -0.25 D preoperatively, with a mean cylindrical value of -4.93 D.
Postoperatively, the spherical refraction in the first group ranged from -7.00 to +1.50 D; the mean cylindrical value was -2.22 D. In the group 2, the spherical refraction ranged from -7.00 to +4.75 D, and the mean cylindrical value was -2.38 D.
The SE improved in both groups; SE improved from -5.89 ± 3.37 to -2.65 ± 2.65 (p<0.001) in the group 1 and from -6.91 ± 1.93 to -2.11 ± 3.01 postoperatively (p<0.001) in the group 2.
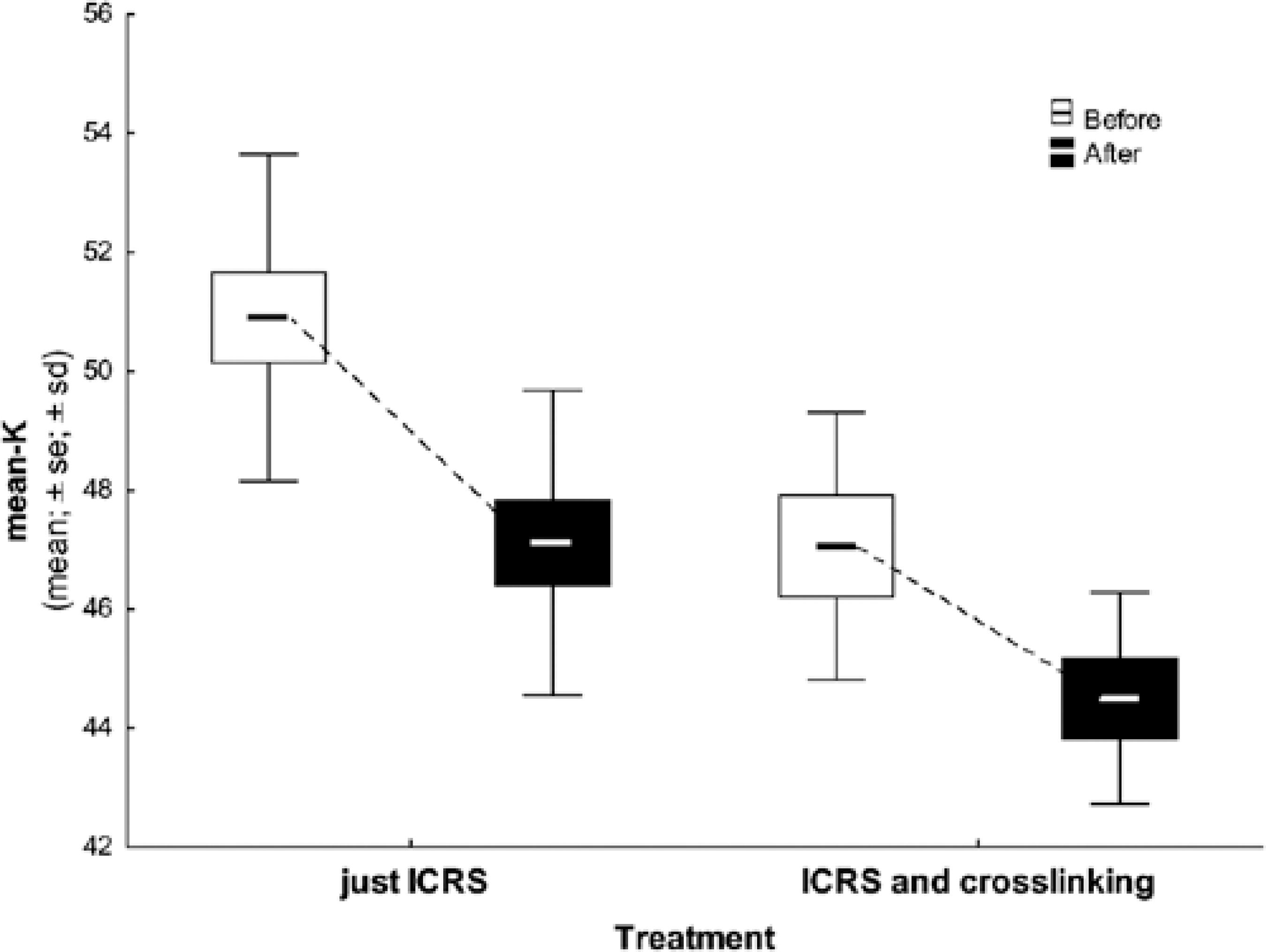
Regarding the keratometric values in the pre- and postoperative topographical analyses, only one implant did not demonstrate a decreased corneal apex value. However, all other patients had an improvement in VA and SE (group 1, p=0.001; group 2, p=0.01). Table 3 and figure 2 show the pre- and postoperative keratometric data.
Table 3 Differences in mean keratometric data between groups
| Treatment | n | Mean | Median | Minimum | Maximum | Standard deviation | P value | |
|---|---|---|---|---|---|---|---|---|
| Mean K pre | ICRSI | 13 | 50.90 | 50.89 | 47.34 | 55.74 | 2.75 | |
| ICRSI + CXL | 7 | 47.06 | 47.15 | 42.61 | 49.72 | 2.24 | 0.018* | |
| Mean K post | ICRSI | 13 | 47.12 | 47.43 | 43.33 | 51.76 | 2.56 | |
| ICRSI + CXL | 7 | 44.50 | 45.61 | 42.03 | 46.05 | 1.78 | 0.768** | |
| Difference pre and post | ICRSI | 13 | 3.78 | 3.99 | -2.29 | 6.27 | 2.10 | |
| ICRSI + CXL | 7 | 2.56 | 2.45 | 0.41 | 5.16 | 1.85 | 0.768** |
*= Student's t-test for independent samples, p<0.05;
**= ANCOVA
VA= visual acuity; ICRSI = intrastromal corneal ring implantation; CXL= crosslinking.
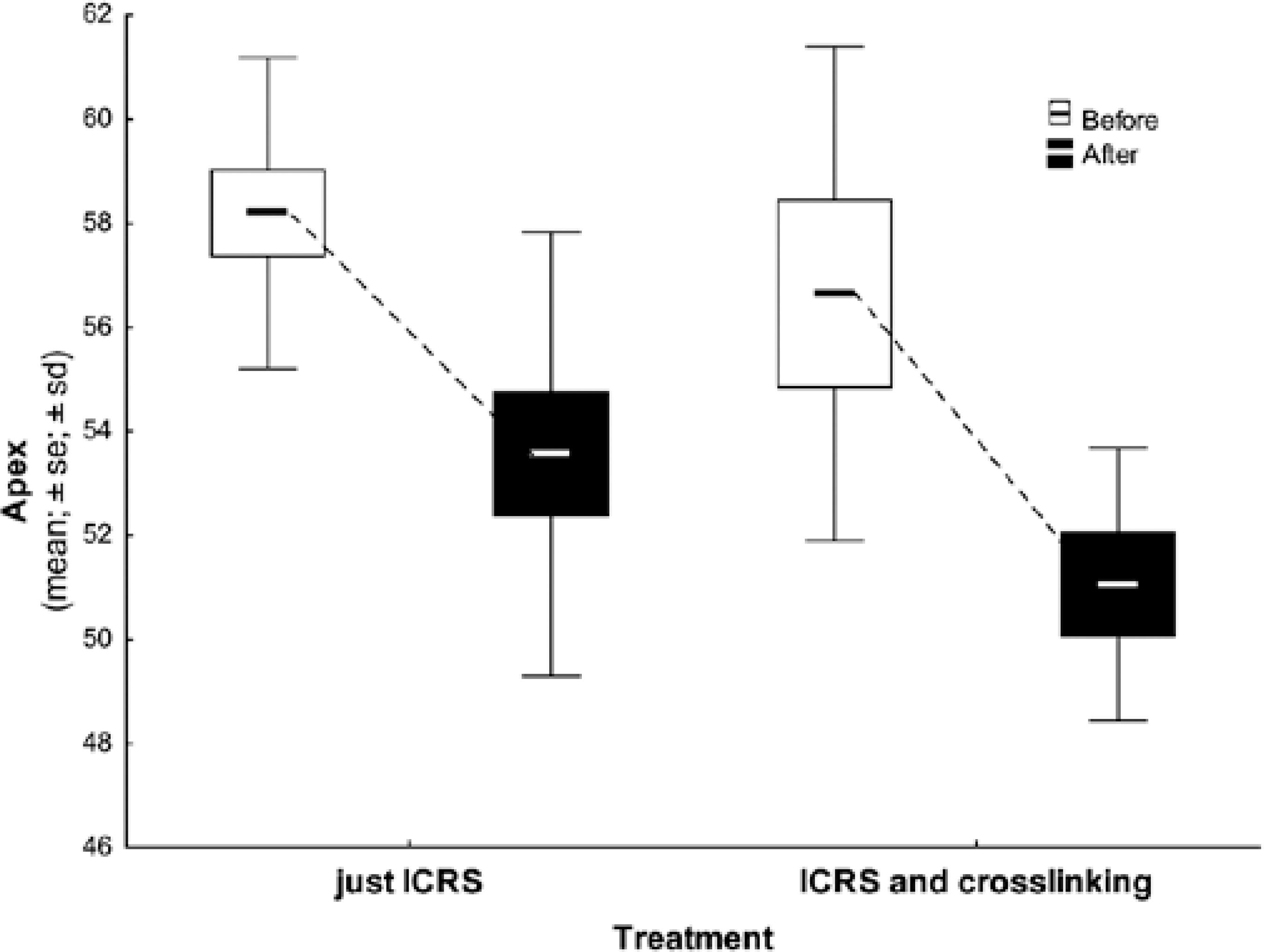
The mean K value of the corneal apex improved after the procedure (group 1, p=0.001; group 2, p=0.007), although there was no significant statistical difference between the groups (Table 4 and Figure 3).
Table 4 Differences in corneal apex between groups
| Treatment | n | Mean | Median | Minimum | Maximum | Standard deviation | P value | |
|---|---|---|---|---|---|---|---|---|
| Apex pre | ICRSI | 13 | 58.19 | 58.18 | 53.03 | 63.40 | 2.99 | |
| ICRSI + CXL | 7 | 56.65 | 57.72 | 48.06 | 62.43 | 4.75 | 0.493* | |
| Apex post | ICRSI | 13 | 53.57 | 52.02 | 47.13 | 64.77 | 4.27 | |
| ICRSI + CXL | 7 | 51.06 | 51.49 | 47.53 | 55.11 | 2.62 | 0.309** | |
| Difference pre and post | ICRSI | 13 | 4.63 | 5.46 | -6.59 | 8.35 | 3.66 | |
| ICRSI + CXL | 7 | 5.59 | 6.23 | -1.38 | 10.43 | 3.67 | 0.309** |
*= Student's t-test for independent samples, p<0.05;
**= ANCOVA
VA= visual acuity; ICRSI= intrastromal corneal ring implantation; CXL= crosslinking.
In the group 2, when comparing the data 3 months after ICRSI, we observed statistical differences in the spherical refractions (p=0.007), astigmatism (p=0.01), SEs (p=0.002), corneal apices (p=0.006), and VAs (p=0.007) (Table 5).
Table 5 Follow-up of the ICRS + crosslinking group after 3 months
| Variable | Pré | Pós 3 m | P value* |
|---|---|---|---|
| VA CC | 20.00 ± 0.00 | 20.00 ± 0 .00 | - |
| VA logMAR pré | 0.56 ± 0.34 | 0.21 ± 0.12 | 0.007 |
| Spheric pré | -4.45 ± 2.18 | -2.33 ± 3.13 | 0.007 |
| Cilindric pré | -4.93 ± 1.03 | -2.30 ± 1.88 | 0.010 |
| SE pré | -6.91 ± 1.93 | -3.38 ± 3.42 | 0.002 |
| K-average pré | 47.20 ± 2.10 | 44.10 ± 4.00 | 0.088 |
| Apex pré | 56.90 ± 4.40 | 52.10 ± 3.00 | 0.006 |
*= paired Student's t-test, p<0.05.
DISCUSSION
The correction of irregular astigmatism caused by primary corneal ectasia is a challenging process. With advancing topographical changes, optical correction becomes ineffective, and the main treatment consists of rigid contact lenses. These provide a uniform surface that neutralizes the myopia and irregular astigmatism associated with keratoconus. When these patients become intolerant to contact lenses, even in the absence of a lesion at the ectasia apex, corneal transplantation is recommended(14).
Several surgical procedures have been proposed as an alternative to penetrating keratoplasty for the treatment of keratoconus, such as photorefractive keratectomy, epikeratoplasty, sectorial keratectomy, and lamellar keratoplasty; however, some of these have yielded disappointing results(2-4). The deep lamellar keratoplasty and penetrating keratoplasty procedures have considerably improved in recent years(15,16). The best spectacle-corrected VA, refractive results, and complication rates have been similar for both techniques, although the nature of complications varied depending on the technique. Deep lamellar keratoplasty is technically more challenging than penetrating keratoplasty; however, it prevents endothelial rejection and may reduce the risk for late endothelial failure(15). Despite its complications, such as the side effects of corticosteroids and allogeneic reactions, the success rate of penetrating keratoplasty is 93%-96%(17,18).
In 2000, Colin et al.(19) described their preliminary results for keratoconus management using ICRSI. Since then, several studies have shown that this was a safe procedure for the correction of corneal ectasias and astigmatism using the manual tunnel dissection technique(19-22). However, several complications related to the manual dissection technique have been observed: epithelial defects, anterior and posterior corneal perforation, superficial placement and displacement of the segment, stromal thinning, extension of the incision to the center of the cornea or close to the limbus, infectious keratitis due to the introduction of epithelial cells in the tunnel, extrusion of the segment, and stromal edema around the tunnel(23, 24). Rabinowitz et al.(25) observed epithelial defects in 50% of patients when using the manual tunnel dissection technique.
A small amount of long-term data is available for ICRS placement, and any effect of ICRSI on disease progression remains uncertain. In a case series of Intacs ICRSI with 3-year follow-up in 13 eyes, significant increases in the average K values were observed between 6 months and 3 years, indicating that disease stabilization was not achieved by ICRSI alone(8).
Intacs alone may not stop progressive keratoconus. Alió et al.(8) found a 1.67 D rise in mean K values between 6 months and 36 months in a series of 13 eyes after ICRSI. This was expected, particularly in cases of rapidly-progressing keratoconus, because the ring insertion does not treat the underlying structural issue of the weakened collagen. Therefore, combining CXL with ICRS insertion in patients with progressive keratoconus is an attempt to ensure stability. It has been proven that CXL, unlike ring insertion, increased the biomechanical rigidity by 4.5-fold. In addition to the crosslinking of the collagen lamellae, the collagen fibril diameter also increases(26).
The literature has not shown significant differences between refractive outcomes among different models with same shapes and sizes for ICRS. Haddad et al. compared Intacs SK ICRS and Keraring SI6 ICRS; both ICRS models significantly improved the visual function in patients with keratoconus with comparable postoperative profiles and no major complications(27).
The first clinical result on the use of femtosecond laser for tunnel creation was reported in 2003 by Ratkay-Traub et al.(28). They evaluated a limited series of 16 eyes and obtained refractive results similar to those observed in patients who had the tunnel constructed manually. Others have also reported a significant improvement in corrected and uncorrected VA and in keratometric values after ICRSI using femtosecond laser(25,29,30). In a study by Coskunseven et al. in 2008(30), 68% of 50 eyes exhibited improved corrected VA. The mean keratometry decreased from 50.6 D to 47.5 D, and the mean SE decreased from -5.6 D to -2.4 D over a year. In our study, we observed an improvement in the corrected VA in 93.7% eyes. The mean keratometry decreased from 50.90 D to 47.12 D, and the mean SE decreased from -5.89 D to -2.65 D over a year in the group that also had ICRSI. When associated to CXL, the mean keratometry decreased from 47.06 D to 44.50 D, and the mean SE decreased from -6.91 D to -2.11 D during the same period. We also found two recipients in the group 1 that did not gain any lines of vision (in one case, the SE changed from -5.00 D to -8.00 D; and in the other, the SE improved from -2.25 to -0.5 D with no gain in VA).
Kubaloglu et al.(29) compared the manual technique with the femtosecond laser and reported a significantly higher rate of epithelial defects when using the manual technique (44% vs. 14%). In this study, authors obtained similar refractive SE and VA results with both tunnel construction techniques and observed that the incidence of complications was significantly lower when using the femtosecond laser technique than that with the manual technique. Moreover, it has been confirmed that using laser for tunnel construction made the procedure easier, quicker, and more comfortable for the patient and surgeon and allowed a more precise corneal dissection at a predetermined depth(25,27). In 2011, Coskunseven et al. described the occurrence of intraoperative complications during ring implantation in 850 eyes using femtosecond laser, including incomplete tunnel formation (2.6%), galvanometer lag error due to system malfunction (0.6%), endothelial perforation (0.6%), incorrect entry into the tunnel (0.2%), and loss of vacuum (0.1%) as well as postoperative complications, such as ring migration (0.8%), corneal melting (0.2%), and infection (0.1%)(28). In our study, no intraoperative or postoperative complications were observed, including in the group that also underwent CXL.
CONCLUSION
In summary, we observed a significant improvement in VA and refractive SE values, decreased curvature of the cone apex in the topographical analysis, and decreased corrected diopters postoperatively. ICRS implants do not ensure control of the ectasia and CXL does not affect visual outcomes in patients who underwent the ICRSI procedure. Furthermore, no postoperative complications were observed with the femtosecond laser and CXL procedures. Both techniques were safe and effective in reducing irregular astigmatism and myopia in patients with keratoconus.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

