Introduction
Epidemiological studies have indicated increasing incidence of dry eye disease (DED) in the worldwide population(1-3). This common ocular condition has multiple causes, which are not entirely understood. The emerging awareness that environmental factors can contribute to DED is supported by some recent studies and reflects differences in cultural traditions and exposure to unfavorable working conditions. In these groups, the impact of environmental factors in DED demands further characterization to develop strategies to reduce its incidence(4,5). Due to the apparent relationship between the aforementioned environmental influences and DED, this disease can be better defined by delineating Environmental Dry Eye Disease (EDED) as a clinical subtype of DED.
We summarize herein our current understanding of environmental causes for DED. However, this review does not deal with some other relevant exogenous factors, such as usage of drugs and alcohol as their effects were recently well addressed(6,7). In addition, we omitted the effect of nutritional factors on DED, which due to its extension and complexity deserves an entire review.
EDED is not only an association between environmental conditions and well-defined ocular surface inflammatory conditions, it is considered as a clinical subtype of DED (Table 1). In EDED cases, the inflammatory conditions and ocular discomfort are followed by changes in tear film composition volume and osmolarity, which may persist even after the individuals are no longer exposed to the related environmental factors. In one example of EDED, following an environmental accidence the symptoms persisted for two years(8-10).
Table 1 Differential diagnosis of Environmental Dry Eye Disease
| Disease | Causes | Clinical features |
|---|---|---|
| Solar keratitis (19) | UV exposure | Keratosis of exposed skin, burning sensation, redness, punctate keratitis |
| Allergic keratoconjunctivitis(10)(20) | Allergy | History of allergy, redness, itching, swelling |
| Floppy eyelid syndrome(21) | Rubbery, redundant upper tarsus | Lid eversion with minimal pressure. Redness, papillary conjunctivitis |
| Corneal hiperalgesia(22) | Up regulated nocioreception triggered by corneal damage | Disparity between signs and symptoms, corneal sensory deficit and decreased sensory nerve population |
| Ligneous conjunctivitis(23) | Impaired mucosal wound healing and fibrosis due to plasminogen deficiency | Chronic membranous conjunctivitis triggered by local trauma |
| Mucous fishing syndrome(24) | Excess of mucous manipulation | Secretion, redness, and foreign body sensation along with signs of epithelial trauma due to discharge of mucus |
| Pseudopemphigoid(25) | History of topical drug exposure. | Redness, tearing, itching, and progressive fibrosis of conjunctiva |
| Chronic blepharitis(26) | Dysfunction of lipid production and secretion and epithelial metaplasia | Lid erythema, greasy crusting secretion. Redness and irritation of the eye Seborrhea in other sites of the body |
| Stevens-Johnsons Syndrome(27) | Autoimmune disease triggered by drug or microorganism | Acute: systemic epithelial bullous swelling. Chronic: ocular surface fibrosis, corneal vascularization, and recurrent epithelial defect |
| Toxic keratoconjunctivitis(28) | Toxic agent traumatic, iatrogenic or factitious contact with ocular surface | Variable, depending on agent and time. Commonly, acute epithelial swelling, redness, and tearing |
EDED is strongly influenced by one or more environmental factor. Additionally, improper diagnosis of toxic keratoconjunctivitis, solar keratitis, allergic keratoconjunctivitis or some other types of ocular surface disease can contribute to EDED. These conditions show similar symptoms and environmental factors on the existing condition further leading to EDED. It is likely that some overlap exists among the mediators of these diseases. This review focuses on some unique environmental factors distinctive of EDED (Figure 1).
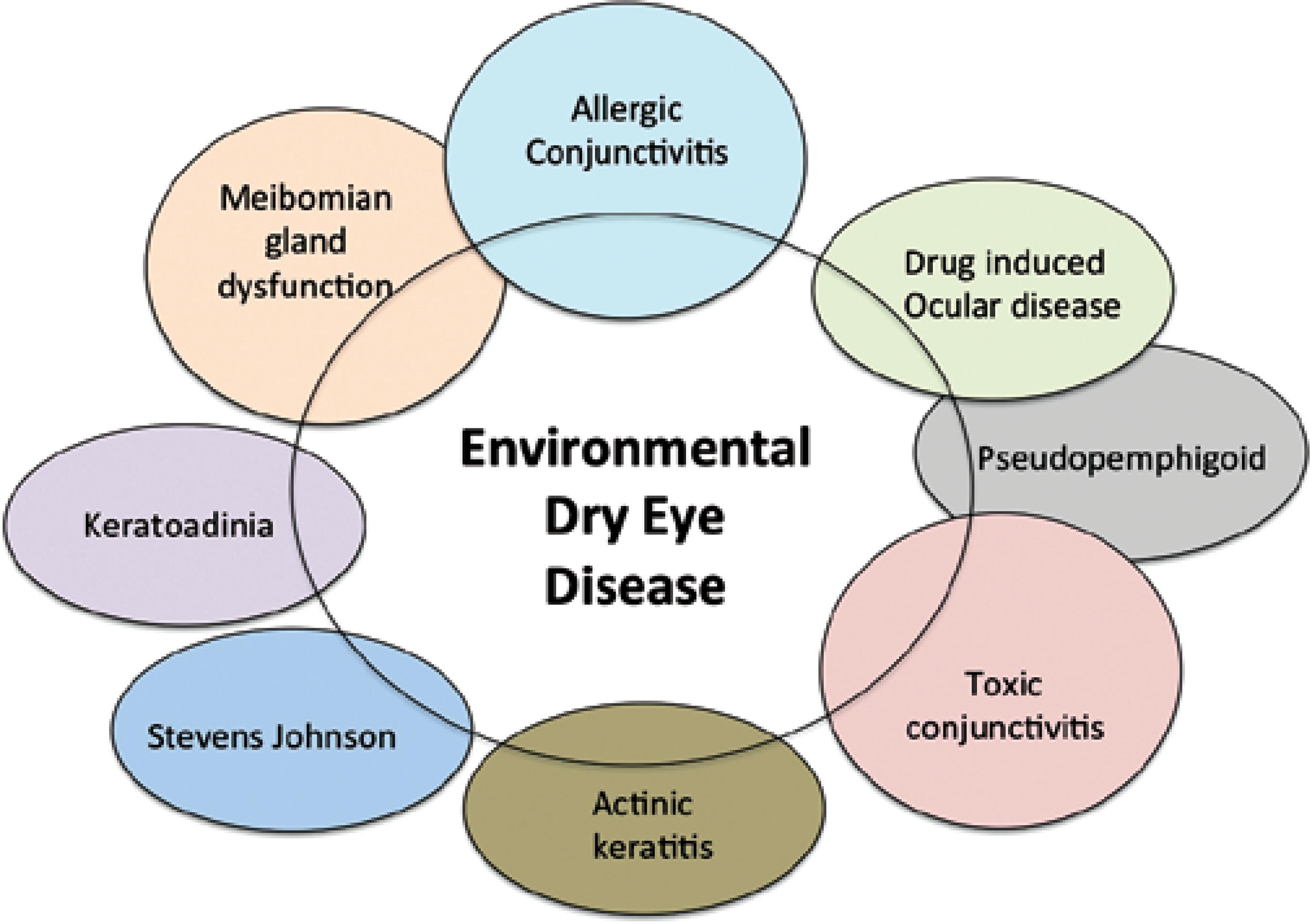
Figure 1 Illustration of conditions whose signs and symptoms may overlap with environmental dry eye disease clinical presentation.
EDED gained relevance based on the recognition that environmental factors can be deleterious to human health and contributes to DED(11,12). The impact of the environment in the pathophysiology of DED has been studied and confirmed in animal models of human DED(13-15).
As indicated, a healthy and pain-free ocular surface depends on identifying and eliminating factors that cause ambient humidity, airflow and purity, and temperature to intolerable levels. Such an undertaking is needed to preserve tear film qualities commensurate with ocular surface health. This is essential to sustain sufficient corneal refractive power, visual acuity, and ocular comfort(5,16,17).
A desiccating environment can lead to increase in tear film evaporation and/or decline in its turn over and clearance. These initial events lead to exposure of the ocular surface to hazardous environmental elements that trigger or exacerbate EDED symptoms. Clinical findings have shown that increased numbers of people are affected by EDED because of exposure to environmental factors (Figure 2).
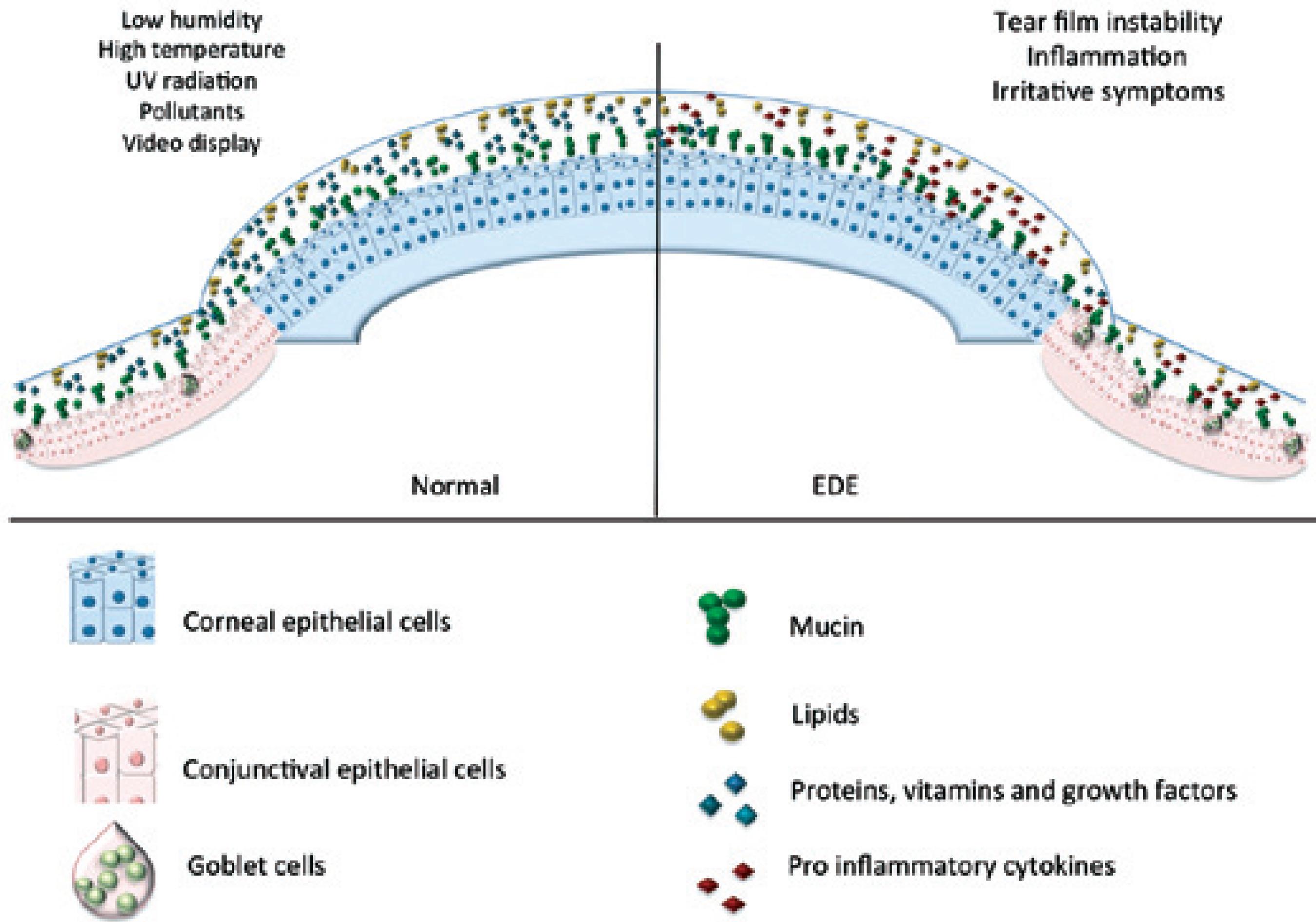
Figure 2 Environmental factors that influence tear film and ocular surface indoors and outdoors (air borne particles, air flow, temperature, ultraviolet rays).
Our purpose herein is to provide a critical appraisal of the clinical and epidemiological evidence indicating that DED is influenced by environmental factors. Secondly, we delineate EDED as a single clinical entity with a unique set of symptoms and clinical findings different from that of either DED or other diseases, such as Sjögren's syndrome, diabetes mellitus or drug induced, allergic conjunctivitis, toxic or irritative conjunctivitis, and actinic keratitis. Moreover, we will describe standard tools used to monitor environmental conditions and discuss their relevance in EDED epidemiological studies. This review enables the health care providers and researchers to identify the environmental risk factors associated with the development and progression of EDED. This initiative intends to help future studies to improve our understanding and care for this possibly common and complex disease.
Environmental Dry Eye Disease (EDED) can be considered a clinical entity that qualifies the definition of DED(18). The most relevant causal factor is environmental exposure, such as pollutants and/or adverse climate. On the other hand, causative factors related to systemic diseases (endocrine, metabolic, nutritional, autoimmune, genetic, viral or neurologic), anti-cholinergic, adrenergic or other drug-related side effects are considered as exclusion factors to maintain EDED as a distinct disease entity within the DED envelope.
EDED differential diagnosis comprises the entities shown in table 1.
Possible non-environmental factors or undiagnosed causes of DED were also considered as differential (for example a suspected Sjögren's syndrome was not investigated to fulfill the criteria). From this perspective, diseases that were labeled as "pollution keratoconjuncitivitis," "computer vision syndrome," and other environmentally related encounters, due to DED clinical presentation would be defined as EDED(29-31). Patients can be afflicted with EDED due to exposure to a variety of environmental stresses. Conditions that elicit this disease are patient dependent(32,33). To establish a minimal normative classification for research and clinical purposes and therapeutic measures, the following environmental factor categories are included:
Indoors: closed ambient like office settings, such as variations in airflow, humidity, time in front of computer and other video displays, and exposure to toxic elements (for example offices, health care facilities, poorly ventilated confinements such as subway stations and other employment areas).
Outdoors: exposure to open areas with extreme temperatures, gases and/or air suspended particles in the desiccating wind, intense UV exposure, agricultural usage of state of the art technology and mechanization, petrochemical industries, urban traffic, and other polluted environments.
Although the clinical signs of EDED can be similar among individuals exposed to either indoor or outdoor environmental factors, the detection preventive, and therapeutic methods are specific for each of these different settings. Additionally, the combined exposure to indoor and outdoor factors is also plausible.
Since the 1960's, exposures to environmental factors such as air pollution had been correlated with ocular surface irritation, resulting in symptoms of hyperemia, swelling, tearing, and dry eye sensation(34).
EDED patients present a broad range of symptoms, the most common being sorrow eyes and visual fatigue. The scores obtained from structured questionnaires have been used for the analysis. One of them is the Ocular Symptom Disease Index (OSDI), which evaluates DED severity rather than EDED(35-37). In order to correlate ocular surface related DED signs with environmental activities, an interesting activity log for DED was recently developed and tested in patients. However, individual differences in pain perception or exposure to environmental hazards in DED initiation were not evaluated(5).
EDED is distinguishable from aforementioned, because it is chronic and is associated with environmental factors. Such clinical findings are identifiable by the clinician/researcher investigating the disease. EDED identification stems from controlled observations about pollution-induced ocular alterations, such as blinking rate, tear film break up time (TFBUT), and corneal epithelia damage(38,39).
Tear film instability is a consistent finding in studies that showed an association between air pollution and ocular surface damage(37,40-42). Such an effect compromises the corneal epithelial barrier function resulting in corneal and conjunctival epithelial chronic injury and inflammation.
A recent study has described a possible early adaptive response to air pollution in which increased levels of air pollution reduce tear film osmolarity and conjunctival goblet cell density(8,37). This negative correlation is indicative of EDED whereby increase in air pollution and/or desiccation are thought to have an early reactive phase followed by a chronic adaptive/metaplastic phase. Clinical findings can help to identify the contributions of exposure time to EDED progression (Table 2).
Table 2 Clinical findings in early and chronic phases of Environmental Dry Eye Disease, compared with non-exposed individuals(8,37,41)
| Clinical findings | Early reactive phase | Chronic adaptive phase |
|---|---|---|
| Symptoms | Variable | Low |
| Tear film osmolarity | Lower | Higher |
| Hyperemia | Present | Present |
| MGD | Present | Present |
| Schirmer test | High | Low |
| TFBUT | Lower | Lower |
| Vital staining | Normal | Altered |
| Conjunctival Goblet cells | Higher number | Lower number |
On the other hand, it is possible that other clinical signs may be associated with the disease, complicating a definitive diagnosis. For example, larger lid opening, lower mucous production, slower blinking rate, and reduced tear film clearance. Such symptoms may be found in different individuals afflicted with different degrees of EDED severity even if they are exposed to the same adverse environmental factors. Future studies are needed to characterize and weigh the individual contributions of commonly observed environmental factors to EDED progression.
Epidemiology of dry eye related to environmental factors
Outdoor EDED risk factors include exhaust emissions from automobiles and industrial facilities common in densely populated cities(43,44). Furthermore, occupational hazards related to large-scale agriculture and sugar cane processing can lead to exposure to gases, particulate matter, UV exposure, and altered microbiota(45,46).
On the other hand, indoor environmental conditions involving low humidity, excessive use of video display units (VDU), and high levels of CO2 can be equally threatening to ocular surface health(4,47-49).
Case-control studies confirm the cause-effect relationship between the indoor or outdoor environmental conditions and the irritant symptoms in exposed individuals(49,50). Also, the individual risk factors are similar to those in other populations afflicted with other types of DED(51). They include aging, females, allergic or autoimmune conditions, and usage of contact lenses(4,48,52-55).
Part of the confusion that persists about EDED recognition is because individuals exposed to high air pollution levels are often at greater risk of developing allergies and present more symptoms(55). Moreover, there is also an association between increase in air pollution and autoimmune diseases, such as rheumatoid arthritis and systemic lupus erythematous(56-58). There is also speculation about the existence of an "urban allergy syndrome" (Table 3)(59). Such individuals have a higher incidence of ocular surface inflammation and DED.
Table 3 Individual and environmental risk factors for Environmental Dry Eye Disease (EDED)
| Risk factors for EDED | |
|---|---|
| Individual | Age, female gender, contact lens usage, lengthy exposure to the hazards (video display, air conditioning), allergies, eye make up, blinking frequency |
| Environmental | Humidity, temperature, high levels of pollutants (particulate matter, CO2, NO2, elemental carbon), regions with heavy automobile transport, pollutant industrial activity, subway stations |
Although the acceptable levels of pollutants are established by national and world agencies (see below), the cut-off limits of the most harmful pollutants and environmental risk factors to the ocular surface capable to induce EDED are unknown. If more than one factor is involved, depending upon their characteristics they may interact at lower threshold values and induce EDED. Accordingly, the detrimental effects of environmental toxic agents on EDED epidemiology are not entirely understood(43,44,60-62).
The impact of urban pollution and environmental toxins on the ocular surface has been evaluated in a few case-control studies. These studies reported a high incidence of ocular discomfort, tear film instability, and ocular surface changes among individuals lived in highly polluted cities of the world (Table 4).
Table 4 Effects of pollution on ocular surface: summary of epidemiological studies
| Author | Year | study design | Local | n | Endpoints | Conclusions |
|---|---|---|---|---|---|---|
| Versura(44) | 1999 | Case-control | Italy | 200 | Schirmer | Abnormal values of BUT 32%, |
| Ferning | Schirmer 40%, and Ferning 45%. | |||||
| BUT | Abnormal impression cytology: | |||||
| Impression cytology | odds ratio 2.66 (IC95% 1.42-5.02) | |||||
| Inflammation | Inflammation: odds ratio 2.27 | |||||
| (IC95% 1.14-2.16) | ||||||
| Gupta(63) | 2002 | Case- control | New Delhi, India | 400 | BUT | BUT (odds ratio: 5.63, IC 95% 2.76-11.46) |
| Schirmer | ||||||
| Lysozyme activity | ||||||
| Saxena(43) | 2003 | Case-control | New Delhi, India | 500 | Symptoms Lysozyme | Positive correlation with symptoms BUT and Schirmer were lower in |
| Rose bengal | study group | |||||
| BUT | No difference in lysozyme | |||||
| Schirmer | and rose bengal | |||||
| Novaes(8) | 2007 | Case-control | Sao Paulo, Brazil | 29 | Impression cytology | Increased goblet cells density in |
| individuals exposed to higher levels of NO2 | ||||||
| Novaes(41) | 2010 | Series of cases | Sao Paulo, Brazil | 55 | Symptoms | Positive correlation with symptoms |
| BUT | Higher incidence of MGD, BUT weak | |||||
| Schirmer | correlation and Schirmer, no correlation with levels of NO2 | |||||
| Malerbi(64) | 2012 | Series of cases | Sao Paulo, Brazil | Meibomian gland evaluation | Increased incidence of blepharitis in high levels of NO2 |
Two studies in New Delhi compared the groups of individuals living inside and outside the metropolitan area(63). A higher frequency of EDED findings with TFBUT <10 seconds, Schirmer Test (ST) <10 mm, and low lysozyme levels in tears were reported among the individuals living within the metropolitan area. The decline in these values correlate with increase in pollutant levels in the metropolitan area compared with the rural area. Another study on 500 volunteers documented a greater frequency of lower ST values and TFBUT values in hospital workers more exposed to traffic derived air pollution(43).
In Italy, patients who presented to an ophthalmological emergency unit with "eye discomfort," reduced ST values and tear film instability were evaluated during the periods of acute rise in air pollution levels, summer and winter. Road traffic, heating system usage, and photochemical smog levels were reported as the main causes of their symptoms. Subjective symptoms were ocular irritation, such as heavy or tired eyes, foreign body sensation, burning, stinging, and photophobia. Also, impression cytology findings in six areas of bulbar and tarsal conjunctiva were altered in 69% of the subjects-49% presented an early loss of goblet cells, 15% showed a total loss of goblet cells without keratinization, and 5% had a total loss of goblet cells with mild keratinization. Women showed higher frequency of symptoms that include ST<10 mm, and higher impression cytology score. In those subjects who lived in more polluted areas (urbanized compared to rural areas), impression cytology documented a greater frequency of keratinization and higher numbers of inflammatory cells (mainly mononuclear cells) in conjunctival scraping scores(44).
Exposure to NO2, traffic derived air pollutant, and ocular surface changes were studied in volunteers in Sao Paulo, Brazil, the largest city in Latin America, and compared with individuals from a countryside area. The individuals living in Sao Paulo showed high levels of NO2 exposure and displayed goblet cell hyperplasia as a result of the chronic insult(8). The same research group analyzed 55 cases of NO2 exposure and found that there is a dose-response relationship between incidence of symptoms and higher prevalence of meibomian gland dysfunction. However, there was a weak negative association with TFBUT and no correlation with ST values(41). Recently another study demonstrated that exposure to high levels of air pollutants canlead to eyelid margin alterations(64). The series of studies on the effects of air pollution on EDED in Brazil were innovative. The pollutant levels were individually measured with portable filter paper for a period of time rather than using broad environmental indices. Together, these findings suggest that life in large and polluted cities cause increased exposure of risk factors toward EDED.
Ultraviolet (UV) radiation is a common risk factor to ocular surface health for unprotected outdoor workers. For instance, climatic droplet keratopathy has been described and correlated with UV exposure(65). Excess exposure to UV has been considered to result in acute tear film instability and induce transitory EDED, however, the reports are contradictory. Not enough evidence is currently available to indicate UV is a risk factor for EDED(66-68). On the other hand, it has been well established that UV is one of the major risk factors in pterygium, a degenerative condition of the ocular surface resulting tear film instability. However, a cause and effect relationship still needs to be determined(69,70).
A large-scale study in Indonesia had shown that agricultural work is not a risk factor for EDED. Type of agricultural activities, amount, and time of exposures, climatic, chemical, and other environmental conditions need to be controlled in future studies to better understand their contributions as possible risk factors. Such an assessment entails delineating involvement of pesticides, fire, and UV irradiation(70).
Indoor environmental contamination also has adverse health effects. The factors are lumped together into a group of signs and symptoms named the "Sick Building Syndrome" (SBS). In the last few decades the symptoms were described in workers in poorly ventilated office buildings. SBS includes non-specific ocular, nose and throat irritation, headache, and respiratory symptoms. Emission of the volatile organic compounds (VOCs) from the synthetic materials used in homes and offices together with other micro environmental variables such as temperature, humidity, lighting and airborne substances can also contribute to EDED. They can cause ocular symptoms, tear film instability, and alterations in ocular surface characteristics of EDED(40,62,71-74). In 1992, Norn described that "sick building" workers have "pollution keratoconjunctivitis" with decline in BUT values and epithelial alterations detected by lissamine green staining(31).
The broad ranges of environmental factors in office ambience associated with the demanding video display unit disrupt ocular surface homeostasis(4,30). In epidemiologic and clinical studies it is important to consider the weight of confounding or summing factors such as allergic conjunctivitis, certain oral medications, BAK preservative eye drops, eye make up, blinking frequency, and contact lens wear.
The prevalence of indoor EDED can be estimated based on ocular discomfort complaints by office building workers. Based on the studies performed using questionnaires, it ranges from 5%-40%(62,75). This large range may be due to the design of questionnaire, types of reported symptoms, inclusion of confounding variables that include contact lens wear, medications, and differences in recall periods. For instance, in 56 European buildings across 9 countries, 39% of the individuals showed the mean prevalence of dry eye symptoms(76).
The anterior ocular surface forms a mucosal interface with large area continuously exposed to the environment. Comfort, proper visual acuity, and cellular maintenance are guaranteed by complex and harmonic interactions of epithelial cells and accessory glands and tear film compounds. Since the anterior ocular surface is the most densely innervated area of the body(77), it is very sensitive to irritants and adverse environmental conditions (Figure 3).
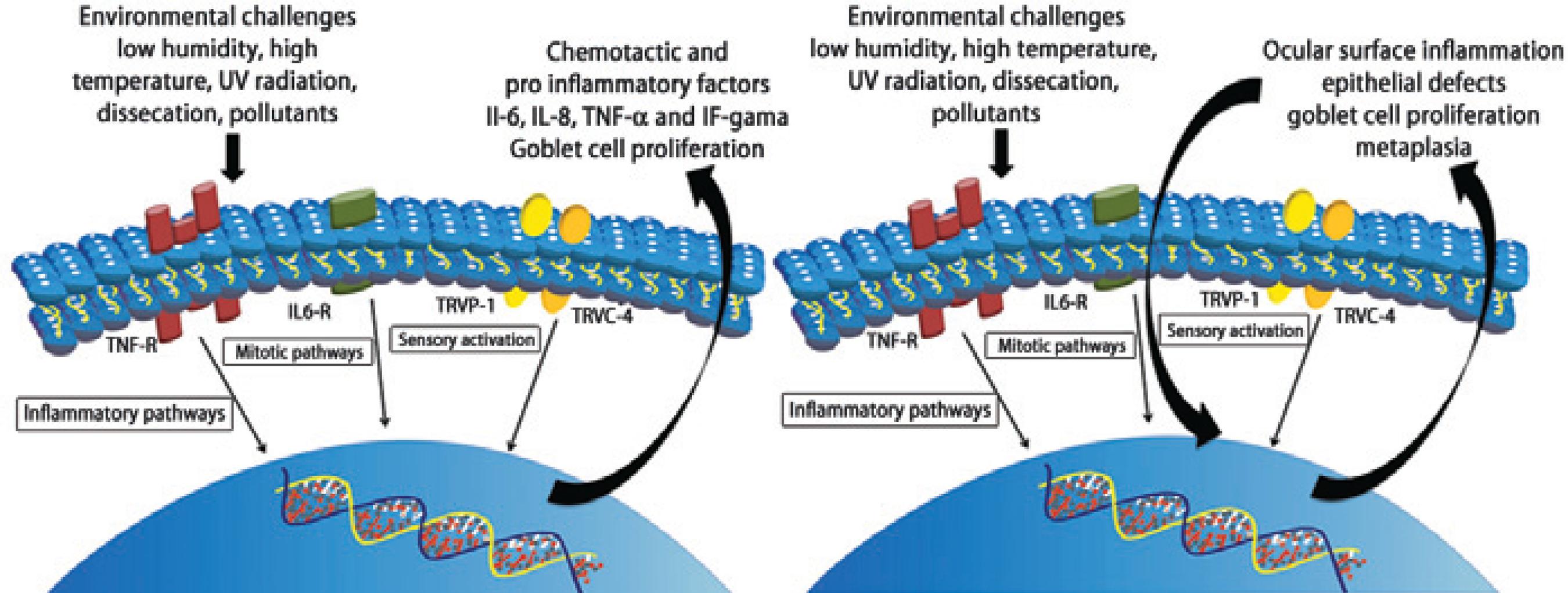
Figure 3 Illustrative steps of pathophysiological mechanisms in the acute phase of EDED at the cellular level
Receptor-induced events that mediate signaling pathway of inflammatory responses resulting EDED symptoms such as decline in tear volume and altered composition are not completely understood. The following questions are need to be answered: to what extent the ocular surface discriminates different types of hazards? What makes the transition between an early/reactive phase to a chronic/ adaptive phase?
Environmental injury might induce increasing expression of cytokines, growth factors and other molecules that mediate specific signaling pathways, and corneal inflammatory and allergic response. During acute and chronic phases, specific cell types, inflammatory mediators, and neurotransmitters are involved(78). Such responses vary depending on the nature and intensity of the stimulus and some mediators have been pointed as major contributors in the process of ocular surface damage related to environmental factors. Members of the transient receptor potential (TRP) channel superfamily, which include subfamilies in corneal epithelial and keratocytes, respond to environmental irritants inducing afferent impulses to the central nervous system(79). Chemical burns in mice induce a specific TRP vanilloid type 1 (TRPV1) channel deregulated inflammatory responses leading to corneal melt and opacification(80). The injury-induced inflammatory and opacification responses resulting from TRPV1 activation were attributed to the up regulation of pro inflammatory and chemo attractive cytokines. TRPV1-induces downstream events by eliciting time dependent stimulation of the mitogen activated protein kinase (MAPK) cascade in epithelial cells and stromal fibroblasts(79,81). Corneal epithelial wound is also accompanied by increased release of mitogens such as, epidermal growth factor (EGF), which induces cell proliferation and migration through activation of a TRP channel in the canonical subfamily identified as TRPC4 and MAPK signaling pathway(82-85).
Flow cytometry analysis of the tear fluid collected from the individuals with atopic keratoconjunctivitis after a conjunctival allergen provocation test presented higher levels of interferon-gamma, IL-6, and a borderline increase in IL-10 after 48 hours. There was a significant difference between provoked and unprovoked eye for the same cytokines: IL-6, IFNγ, and IL-10(86). We speculate that individuals exposed to air pollution or other hazardous stimuli can elicit the similar inflammatory cascade during acute phase and lead to EDED clinical presentation, similar to other ocular surface inflammatory diseases, such as AKC(78).
Goblet cell hyperplasia results from exposure to high levels of air pollution in the urban population(8). This is due to chronic exposure to air pollution by human nasal and respiratory mucosal surfaces that are considerably similar to conjunctiva(84,87-89). However, studies in mice exposed to a desiccating environment showed the opposite response. The differences between the human and mouse may be explained by species-specific responses, complexity of the trigger (humans are frequently exposed to combined factors, such as pollutants and adverse climate), and/or observations collected at different time-points in the disease progression of both the species.
In vitro models are useful to simulate hazardous conditions and assess their effects on the ocular surface at the molecular level. In this regard, the observation that particulate pollutants disrupt meibomian gland lipid structure and consequently the tear film organization was reproduced using benzalkonium chloride (BAK) and quartz particles(90). This study suggested that BAK affects the surface activity of meibomian lipids and quartz particles adsorbed to meibomian lipids, by removing them from the air/water interface. The authors proposed that a similar mechanism accounts for the effect of particulate pollutants on the tear film lipid layer(90).
The possibility of measuring specific effects of air pollutants and exposure to other environmental hazards on ocular surface integrity and health will identify the individuals with pathologic correlations to EDED. A recently described method is proven to be useful for this purpose. It comprises a filter paper in a small chamber attached to a belt or other piece of clothing (Figure 4). Air pollutants deposited on filters after different times were eluted and measured(41). In this direction, a better understanding of EDED inducing factors and underlying mechanisms can be achieved. Such insights will help the development of more efficient preventive and therapeutic strategies.
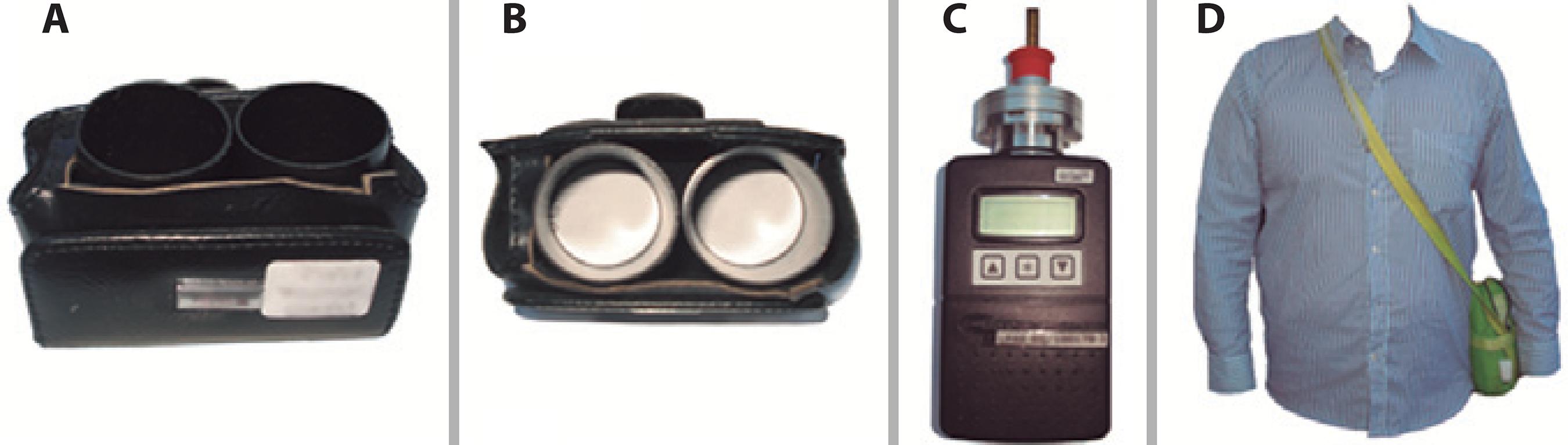
Figure 4 A) Double passive NO2 sampler containing a cellulose filter (Energetica, Rio de Janeiro, Brazil) impregnated with an absorbent solution of 2% triethanolamine, 0.05% o-methoxyphenol, and 0.025% sodium metabisulfite inside a small plastic tube with one of its extremities open to ambient air. The nitrite produced during sampling is determined colorimetrically by reacting the with sulfanilamide and 8-anilino-1-naphthalene-sulfonic acid (ANSA) and monitoring the absorption at 550 nm wavelength; B) Superior view of the NO2 sampler; C) Portable gravimetric impactor with a flow rate of 1.8 L/min. Air is aspirated into the impactor by a pump, PM is retained by a filter, and the particles in suspension in the air are measured gravimetrically; D) Portable sampler carried in a small bag by a research subject.
Environment monitoring tools
The environment is being polluted by industrial waste, automobile and truck exhaust fumes, burning of coal and fossil fuels as well as chemical manufacturing. Air pollution can even come from small-scale every day indoor activities, such as dry cleaning, degreasing, and painting. These activities accumulate gases and particles that come incontact with our mucosal tissues, especially the anterior ocular surface.
There is an increasing demand for environmental health indicators capable of measuring the amount of chemical, climatic, and physical hazards. Since 1987, World Health Organization (WHO) published guidelines for air quality are reviewed periodically(91,92). Similar guidelines, some with differences in items monitored or cut-off levels are provided by the Environmental Protection Agency (EPA) from the USA and other national or continental agencies (Table 5).
Table 5 Websites of agencies that provide guidelines and databases related to the environmental parameters in different regions of the world
| Agency | Website | Country/area |
|---|---|---|
| European Space Agency | http://www.earthobservations.org/geoss_cl_ph.shtml | Global |
| United Nation Economic Commission for Europe | http://www.unece.org/env/europe/monitoring/iandr_en.html | Global |
| World Health Organization | http://www.who.int/ipcs/publications/ehc/ehc_numerical/en/index.html | Global |
| Environmental Protection Agency | http://www.epa.gov/oaqps001/montring.html | USA |
| Health Canada | http://www.hc-sc.gc.ca/ewh-semt/air/in/res-in/index-eng.php | Canada |
| Instituto Nacional de Metereologia do Brasil | http://www.inmet.gov.br/portal/ | Brazil |
| Companhia Ambiental do Estado de São Paulo | http://www.cetesb.sp.gov.br/ | São Paulo State, Brazil |
| Ministry of the Environment Government of Japan | http://www.env.go.jp/en/ | Japan |
| Ministry of Environmental Protection | http://english.mep.gov.cn/ | China |
| Department of Sustainability, Environment, Water, Population and Communities | http://www.environment.gov.au/ | Australia |
| Environment Agency | http://www.environment-agency.gov.uk/ | United Kingdom |
Among outdoor environmental parameters, climatic variables including temperature, atmospheric precipitation, humidity and UV radiation, and air pollutants including particulate matter (PM), CO2, NO2, O3 and SO2 were correlated with ocular and systemic diseases(92-94). As mentioned above, the indoor pollutants are same as the more commonly monitored climatic parameters and they include include pollen, tobacco smoke elements, mold, pesticides, carbon monoxide, formaldehyde, and lead among others(95).
Those indicators are also monitored by national and international health and/or environmental agencies. They are then applied in health analysis studies to correlate diseases with epidemiology, governmental policy directives, and economic studies(12,96,97).
This approach supports analysis of the health status of individuals or groups exposed to the above-indicated environmental risk factors. The results guide public health policies and preventive care(94,98-100). They also help comparing environmental hazards in different areas or countries to promote actions that may reduce their effects(101).
The information collected by the international or governmental agencies are available for public consulting(102). These agencies work together to adopt similar measurement standards, units, and methods, and make the data available to the public through web databases and annual publications(91).
Data on air quality or climate could be used to correlate with clinical observations of incidences of EDED in different cities or regions. However, the daily range of pollutants, hazardous agents, confounding factors, and individual variable time-exposure to any of the studied agents can be too large to precisely identify agents responsible for higher EDED incidence in a certain area. These limitations may make it difficult to draw meaningful conclusions. To avoid that, study designs are required to include homogeneous groups with similar habits and comparable exposure times. One report had described that children living close to three petrochemical companies in Nigeria have tetraethyl lead and black carbon tear film(29). Similar studies are necessary to identify the specific pollutants responsible for a cause and effect relationship between an environmental factor and EDED.
Conclusions
For several years, environmental factors have been known to be associated with DED. A more extensive and detailed analysis of the association between environmental factors and DED suggest that EDED as a DED subtype will aid efforts to pinpoint different factors responsible for this disease. To this end, we propose that combined clinical and laboratory studies can help identify different environmental factors that induce EDED. At this point, we were able to summarize evidence supporting the association of specific environmental hazards such as pollutants and adverse outdoor and indoor environmental factors with EDED.
DED clinical findings and symptoms resulting from environmental factor mediated anterior ocular surface receptor activation induce pro inflammatory cytokines, chemo attractant expression, and elaboration of extracellular stromal matrix due to increases in myofibroblast expression. These mechanisms induce tear film instability, stromal immune cell infiltration, and disruption of lacrimal gland mediated tear film volume and composition. Constant exposure to environmental factors may allow the clinician to distinguish between acute and a chronic phase of the disease.
Environmental data monitoring and safety limits obtained from international or governmental agencies may help clinicians to associate DED disease stages with environmental factor exposure. Researchers may be aided in identifying relevant stress to apply to their different model systems to pinpoint the mechanisms mediating responses underlying EDED.
Unfortunately, except for using protective equipment to counter specific hazardous environmental agent stresses, EDED treatment is limited to the same medications and interventions available for other types of DED.(103) Given this limitation, it remains important to promote novel investigative interventions to treat or minimize EDED damage.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

